Fig. 5.
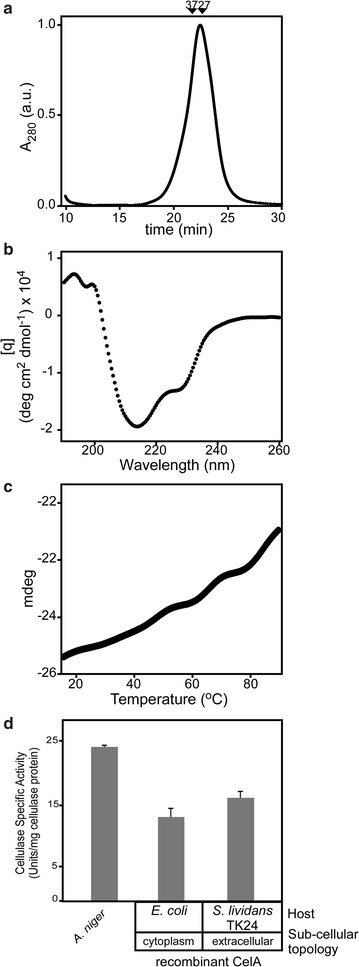
Physical and functional characterization of S. lividans-secreted CelA. a Size exclusion chromatography of recombinant purified CelA. Arrows indicate migration positions for 35 kDa (CesAB/EspA) and 27 kDa (CesAB) [39]. b Circular dichroism spectrometry. 5 µM or protein in buffer (5 mM MOPS pH 7.5, 5 mM NaCl, 1 mM DTT) 20 °C was analyzed using a 190–260 nm wavelength scan. c Thermal denaturation curves monitored by circular dichroism. Purified CelA (5 µM) in buffer (5 mM MOPS pH 7.5, 5 mM NaCl, 1 mM DTT) was exposed to gradual temperature rise and changes in ellipticity were monitored at 213 nm, as described [22]. d Biochemical activity of S. lividans-secreted CelA. Cellulase activity by CelA (20 µg/mL) was determined by hydrolysis of carboxymethyl cellulose (CMC) (see “Methods”). The activity of CelA secreted and purified from S. lividans was compared to CelAHis6 produced in E. coli and to a commercial preparation from A. niger of 24 U/mg total protein estimated to contain ~ 15 µg of unknown cellulases. n = 3; values represent mean ± SD
