Abstract
Impulsive personality traits are heritable risk factors and putative endophenotypes for addiction and other psychiatric disorders involving disinhibition. This study examined the genetic basis of impulsive personality traits, defined as scores on the Barratt Impulsiveness Scale (BIS-11) and the UPPS-P Impulsive Behavior Scale (UPPS-P). In 983 healthy young adults of European ancestry, the study examined genetic variation in relation to a combined phenotype of seven subscales based on high phenotypic intercorrelations. The study first tested 14 a priori loci that have previously been associated impulsive personality traits or closely related constructs. Second, the study included an exploratory genome-wide scan (i.e., GWAS), acknowledging that only relatively large effects would be detectable in a sample size of ~1000. A priori SNP analyses revealed a significant association between the combined impulsivity phenotype and two SNPs within the 5-HT2a receptor gene (HTR2A; rs6313 and rs6311). Follow-up analyses suggested that the effects were specific to the Motor and Non-planning subscales on the BIS-11, and also that the two loci were in linkage disequilibrium. The GWAS yielded no statistically significant findings. This study further implicates loci within HTR2A with certain forms of self-reported impulsivity and identifies candidates for future investigation from the genome-wide analyses.
Keywords: Impulsivity, genetics, endophenotype, personality traits, serotonin
1. Introduction
Despite extensive evidence from twin studies that genetic factors strongly influence addictive disorders (Agrawal and Lynskey, 2008, Goldman et al., 2005) and other disorders of disinhibition (e.g., attention-deficit/hyperactivity disorder [ADHD], borderline personality disorder; Faraone et al., 2005; Distel et al., 2008), the specific genes and polymorphisms responsible have been elusive (Schuckit, 2014). A promising approach to identify the genetic bases of polythetic disorders like addiction is the investigation of endophenotypes, or heritable phenotypes that are putatively simpler in genetic architecture and lie between genetic variation and a psychiatric disorder (Gottesman and Gould, 2003). Endophenotypes may shed light on the etiology of psychiatric disorders by identifying loci that are relevant to both the endophenotype and the disorder. Furthermore, these endophenotypes may ultimately be helpful to improve treatment or prevention efforts (for a full review, see MacKillop and Munafò, 2013).
One broad phenotype that has been consistently linked to psychiatric disorders involving self-regulatory deficits is impulsivity (Amlung et al., 2016; de Wit, 2009; MacKillop et al., 2011). Impulsivity refers to a family of constructs that can be broadly categorized into three primary domains: impulsive personality traits (i.e., self-reported impulsive tendencies), poor response inhibition (i.e., inability to inhibit a prepotent response on experimental tasks), and maladaptive decision making (e.g., preferences for smaller immediate rewards over larger delayed rewards). Although conceptually related, these forms of impulsivity are largely quantitatively distinct from one another (MacKillop et al., 2016; Reynolds et al., 2006).
Here, we focus on measures of impulsive personality traits from an investigation into the latent phenotypic structure of diverse measures of impulsivity. In that study, several measures of impulsivity aggregated into the three aforementioned domains and there was limited overlap between the domains (MacKillop et al., 2016). With regard to impulsive personality traits, MacKillop et al. (2016) included three subscales of the Barratt Impulsiveness Scale, Version 11 (BIS-11) (Patton et al., 1995) and five subscales of UPPS-P Impulsive Behavior Scale (UPPS-P) (Cyders et al., 2007; Whiteside and Lynam, 2001), and found that all three subscales of the BIS-11 and four of the five subscales of the UPPS-P contributed unique variance to an impulsive personality trait factor. The Sensation Seeking subscale on the UPPS-P did not load on the factor. The current study focuses explicitly on impulsive personality traits, not the other two domains, because they constitute quantitatively distinct phenotypes.
Both the BIS-11 and UPPS-P are reasonable choices for use as endophenotypes because they meet most of the criteria proposed for identifying endophenotypes (Flint and Munafò, 2007; Gottesman and Gould, 2003). For example, elevations on these measures are associated with risk-taking behaviors, addictive disorders, and other psychopathology (Berg et al., 2015; Coskunpinar et al., 2013; Stanford et al., 2009) and they show robust evidence of heritability (47–63%; Gustavson et al., 2014; Niv et al., 2012; Seroczynski et al., 1999). Furthermore, impulsive personality traits have been found higher in siblings of chronic stimulant users than controls, but highest in the chronic stimulant users, suggesting that impulsive personality traits are an endophenotype for stimulant dependence that may be exacerbated by chronic drug exposure (Ersche, Turton, Pradhan, Bullmore, & Robbins, 2010). Association studies have implicated several genetic loci with scores on the BIS-11 and UPPS-P (see Table 1), most notably identifying genes involved in dopaminergic and serotonergic neurotransmission. These include DAT1 (Forbes et al., 2009; Paloyelis et al., 2010), DRD4 (Schilling et al., 2014; Varga et al., 2012), ANKK1 (Doran and Trim, 2013; Limosin et al., 2003), COMT (Soeiro-De-Souza et al., 2013; Varga et al., 2012), HTR1A (Benko et al., 2010), HTR1B (Varga et al., 2012), HTR2A (Preuss et al., 2001; Racine et al., 2009), SLC6A4 (Racine et al., 2009; Sakado et al., 2003), and MAOA (Chester et al., 2015). In addition, associations have been reported with variants in BDNF (Su et al., 2014), OPRM1 (Pfeifer et al., 2015), GSK3β (Jiménez et al., 2014), VDR (Wrzosek et al., 2014), NRXN3 (Stoltenberg et al., 2011), and SNAP-25 (Németh et al., 2013). Yet, as we have discussed before (Hart et al., 2013), the loci identified in the aforementioned studies have also exhibited failure to replicate, and some have yielded opposing effects (e.g. Congdon et al., 2008; Eisenberg et al., 2007; Forbes et al., 2009; Jakubczyk et al., 2012; Paloyelis et al., 2010; Roiser et al., 2007; Varga et al., 2012). Many of these studies have used small sample sizes (Table 1, median n = 192) and have had relatively modest genomic scope. Furthermore, many of these studies included individuals with current substance use disorders, which complicates the interpretation because extended drug use can increase measures of impulsive personality (e.g., Quinn et al., 2011). Studying an endophenotype in healthy adults without histories of addiction allows investigators to study normal variation in a trait, without the confounding influence of drug use or psychiatric symptomatology. Finally, the previous studies did not systematically assess associations using multiple measures of impulsivity simultaneously to capture overlapping phenotypes.
Table 1.
A priori loci previously associated with impulsive personality traits
| Chr | Locus | Gene | Risk Allele | Measure | Sample Size | Reference |
|---|---|---|---|---|---|---|
| 3 | rs1732170 | GSK3β | T | BIS-11 Attentional | 188 | Jiménez, 2014 |
| -- | total, Motor, Non-planning | |||||
| 3 | rs334558 | GSK3β | G | BIS-11 Attentional | 185 | Jiménez, 2014 |
| -- | total, Motor, Non-planning | |||||
| -- | BIS-11 total | 884 | Dick, 2013 | |||
| 5 | 3′UTR VNTR | DAT1 | 9-repeat | BIS-11A total | 68 | Paloleyis, 2010 |
| 9-repeat | BIS-11 total | 86 | Forbes, 2009 | |||
| -- | BIS-11 total | 86 | Congdon, 2008 | |||
| 5 | rs6295 | HTR1A | GG | BIS-11 total, Motor, Attentional | 724 | Benko, 2010 |
| -- | Non-planning | |||||
| -- | BIS-11 total | 687 | Varga, 2012 | |||
| 6 | rs13212041 | HTR1B | AA | BIS-11 total | 686 | Varga, 2012 |
| 6 | rs1799971 | OPRM1 | G | UPPS-P Premeditation | 214 | Pfeifer, 2015 |
| -- | Negative Urgency, Perseverance | |||||
| -- | BIS-11 total | 7641 | Laas, 2015 | |||
| 11 | exon 3 VNTR | DRD4 | Short form | BIS-11 total | 192 | Schilling, 2014 |
| Short form | BIS-11 total | 686 | Varga. 2012 | |||
| Long form* | Latent IPT construct | 298 | Carver, 2014 | |||
| -- | BIS-11A total | 68 | Paloyelis, 2010 | |||
| -- | BIS-11 total | 86 | Congdon, 2008 | |||
| -- | BIS-11 total | 86 | Forbes, 2009 | |||
| -- | BIS-11 total, Attentional, Motor, Non-planning | 168–185 | Eisenberg, 2007 | |||
| 11 | rs6265 | BDNF | T | Latent IPTconstruct | 298 | Carver 2014 |
| T | BIS-11 Attentional | 138 | Su 2014 | |||
| 11 | rs1800497 | ANKK1 | T | UPPS-P NU | 121 | Doran, 2013 |
| -- | BIS-11 total | 683 | Varga, 2012 | |||
| -- | BIS-11 total, Attentional, Motor, Non-planning; EIQ | 168–185 | Eisenberg, 2007 | |||
| C | BIS-10 total | 92 | Limosin, 2003 | |||
| T* | Latent IPT construct | 298 | Carver, 2014 | |||
| 12 | rs2228570 | VDR | CC | BIS-11 total, Attentional | 1482 | Wrzosek, 2014 |
| -- | Motor, Non-planning | |||||
| 13 | rs6313 | HTR2A | T | BIS-11 total | 344 | Racine, 2009 |
| -- | BIS-11 total | 284 | Jakubczyk, 2012 | |||
| 13 | rs6311 | HTR2A | GG | BIS-11 total | 135 | Preus, 2001 |
| 14 | rs11624704 | NRXN3 | C | BIS-11 total, Attentional | 439 | Stoltenberg, 2011 |
| -- | Motor, Non-planning | |||||
| 17 | 5-HTTLPR | SLC6A4 | Short form | BIS-11 total | 319 | Racine, 2009 |
| Short form | BIS-11 total, Attentional | 123 | Sakado, 2003 | |||
| -- | Motor, Non-planning | |||||
| Short form** | BIS-11 total | 374 | Paaver, 2007 | |||
| Short form* | Latent IPT construct | 303 | Carver, 2011 | |||
| -- | BIS-11 total | 683 | Varga, 2012 | |||
| -- | BIS-11 total, | 30 | Roiser, 2007 | |||
| Attentional, Motor, Non-planning; scores | ||||||
| 20 | rs3746544-rs1051312 | SNAP25 | T-T haplotype | lower BIS-11 total IVE-I | 869 | Németh, 2013 |
| 22 | rs4680 | COMT | AA | BIS-11 Non-planning | 82 | Soeiro-De-Souza, 2013 |
| -- | total, Attentional, Motor | |||||
| AA | BIS-11 total | 680 | Varga, 2012 | |||
| -- | BIS-11A total | 68 | Paloyelis, 2010 | |||
| -- | BIS-11 total | 86 | Forbes, 2009 | |||
| GG | Latent IPT construct | 298 | Carver, 2014 | |||
| X | rs1465108 | MAOA | A | UPPS-P Negative Urgency | 277 | Chester, 2015 |
Note. VNTR = variable number tandem repeat; IPT = impulsive personality traits. BoldingUrgency indicates loci were genotyped (or imputed) and assessed in the current study. Unless otherwise noted, risk alleles were associated with higher scores on the measures listed (“--” indicates that no relationship was found).
The effects were identified in an interaction with childhood adversity relating to higher latent IPT scores.
The effects were identified in an interaction with low platelet monoamine oxidase activity relating to higher BIS-11 total scores.
315 males and 449 females analyzed separately.
The present project sought to address some of these limitations by investigating impulsive personality traits in a comparatively large sample of healthy, non-drug-abusing individuals (MacKillop et al., 2016), using a wide array of loci. Furthermore, we used a multivariate approach based on evidence that these phenotypes are correlated (MacKillop et al., 2016) and because multivariate methods can detect effects when only one of the variables is associated with a genetic locus (Galesloot et al., 2014). This allowed us to estimate both overall relationship with impulsive personality traits as well as a more fine-grained assessment of associations with individual subscales. The study used a hierarchical approach, first testing a priori loci, explicitly prioritizing loci that had previously been reported as significantly associated in the peer-reviewed literature of impulsive personality traits. Within this first set of analyses we also tested three loci that a recent GWAS found were associated with Neuroticism and Conscientiousness (Lo et al., 2016), two facets of personality closely related to impulsive personality traits (Whiteside and Lynam, 2001). Second, for completeness, we report an atheoretical genome-wide scan (i.e., GWAS), acknowledging that only relatively large effects would be detectable in a sample size of ~1000. Given the paucity of genome-wide studies in this area, this aim was intended to expand the genomic scope to detect previously unreported large magnitude associations, to inform hypotheses in future studies, and to avoid contributing to publication bias in the literature (e.g., Munafò et al., 2004).
2. Methods
2.1. Participants
Full phenotyping methods are provided in MacKillop et al. (2016). In brief, participants were recruited at two sites (Athens, GA and Chicago, IL). Inclusion criteria were English fluency, age 18 – 30 years, and self-reported Caucasian race and non-Hispanic ethnicity to minimize population stratification (Hutchison et al., 2004). Exclusion criteria were scores >12 on the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993) or the Drug Use Disorders Identification Test (DUDIT; Berman et al., 2005). In addition, all participants were screened for recent alcohol or drug use via breathalyzer or urine drug test before testing. A further exclusion criterion was treatment over the last 12 months or self-reported current need for treatment for: depression, bipolar disorder, general anxiety, social anxiety, post-traumatic stress disorder, obsessive compulsive disorder, panic attacks/disorder, phobia, schizophrenia spectrum disorders, anorexia, bulimia, or binge eating. We did not exclude ADHD because while heavy drug use introduces environmental exposure that can increase impulsivity (de Wit, 2009; Quinn et al., 2011; Quinn and Harden, 2013), impulsive personality traits and ADHD are related and likely have overlapping heritability without confounding environmental exposure (Berg et al., 2015; Jepsen et al., 2017). Participants completed assessments individually in a behavioral laboratory. DNA was collected via a saliva sample for DNA collection in an Oragene DNA kit (DNA Genotek Inc., Kanata, ON, Canada).
2.2. Phenotypes
We used the Barratt Impulsiveness Scale, Version 11 (BIS-11), a 30-item measure (Patton et al., 1995) with three second order factors: Attentional, Motor, and Non-planning, and four subscales from the 59-item measure, the UPPS-P Impulsive Behavior Scale (UPPS-P) (Cyders et al., 2007; Whiteside and Lynam, 2001): Negative Urgency, (lack of) Premeditation, (lack of) Perseverance and Positive Urgency. The 5th scale of the UPPS-P, Sensation Seeking, was not included because it was not strongly correlated with the other impulsive personality subscales (MacKillop et al., 2016). Likewise, we did not test loci associated with Extraversion from the recent GWAS (Lo et al., 2016), because Extraversion is most related to Sensation Seeking (Whiteside and Lynam, 2001). Demographic characteristics including sex, age, race, and income were recorded.
2.3. SNP Genotyping and Quality Control
Genotyping was performed using the Illumina PsychArray BeadChip platform, which characterizes ~600,000 SNPs and has been optimized to capture the maximum amount of information about common variation. Quality control filtering was implemented in PLINK v1.9 (Chang et al., 2015). Autosomal SNPs were filtered for call rates < 98%, Hardy-Weinberg Equilibrium (HWE) violations of p < 1 × 10−6 and MAF < 5%. After filtering 437,652 SNPs remained for imputation. Imputation of missing genotypes and of additional SNPs was performed with IMPUTE2 v.2.3.1 (Howie et al., 2009) using the 1000 Genomes Phase 3 b37 reference panel (1000 Genomes Consortium, 2015). Imputed SNPs were excluded for exhibiting an information score of < .3 (Marchini and Howie, 2010), MAF < 5%, HWE violations of p < 1 × 10−6, missingness > 5%, and multiallelic status. Imputed SNPs with confidence < .9 were set to missing. Of the 3 a priori loci from the recent GWAS of personality (rs3814424, rs6981523, rs9611519; Lo et al., 2016) and the 15 a priori loci from prior impulsive personality trait research (see Table 1), two were excluded for excessive missing values (rs1051312, rs334558), one was excluded for poor imputation accuracy (rs3746544), and one was excluded because it was on the X chromosome (rs1465108). VNTR loci were not genotyped and therefore were not included in this study. Following quality control, 14 a priori loci and 4,887,762 genome-wide SNPs were available for association analysis.
2.4. Participant Quality Control
1,000 participants had valid genotyping data (call rates > 98%, inbreeding coefficient absolute value < .02, concordant self-reported sex and X-chromosome determined sex) and satisfied the inclusion/exclusion criteria. To correct any self-reported race that is misclassified as Caucasian, principal components analysis (PCA; Price et al., 2006) was conducted. Two population outliers were identified and removed by visual inspection of the principal components plot (see Figure S1). 13 participants were excluded for missing one or both measures of impulsive personality traits. Finally, participants were assessed for cryptic relatedness (Yang et al., 2011), and two were removed for relatedness > .05, leaving a final sample of 983 unrelated European-ancestry participants (Table 2).
Table 2.
Participant characteristics (N = 983)
| Variable | %/Mean (SD)/Median |
|---|---|
| Age | 21.65 (3.30) |
| Sex | 62.2% Female |
| Income | $60,000 – $89,999 |
| Years of education | 14.53 (2.21)1 |
Note.
N = 982.
2.5. Data Analysis
The internal reliability coefficients and the interrelationships among the UPPS-P and BIS-11 subscales were calculated. Phenotypic variables were standardized to Z-scores prior to the multivariate genetic analyses. Covariates for the study were ascertained via a multivariate linear mixed model (MLMM) of the impulsive personality subscales with four covariates: sex, age, income, and site (i.e., Chicago, IL or Athens, GA). Each candidate covariate was examined as a fixed factor, while the other three covariates were included as control variables and only variables which were significantly associated in the combined models were included as covariates in subsequent analyses. Genome-wide Efficient Mixed Model Association (GEMMA) software (Zhou and Stephens, 2012) was utilized to examine the MLMM associations between the loci from each strata and the impulsive personality subscales. The MLMM accounts for the cryptic relatedness among individuals, which is modeled out as a random effect (i.e., the genetic correlation between individuals). To manage type I error inflation, for the first prioritized subset (14 a priori loci), a Benjamini-Hochberg FDR correction was applied to the resultant p-values from the analyses (Benjamini and Hochberg, 1995). For the atheoretical genome-wide scan, SNPs were examined for genome-wide significance at a nominal p-value less than 5 × 10−8, as this is a consensus within the field for valid genome-wide significance (e.g., Pe’er et al., 2008). Any significant multivariate relationships were unpacked by examining linear relations among the significant loci and each individual subscale. Because GWA analyses comprised a large panel of SNPs, the top 50 genome-wide loci were also reported to avoid excessive type II error rate and inform future inquiries.
3. Results
3.1. Preliminary Analyses
A correlation matrix of the impulsive personality subscales (including internal reliabilities) can be found in Table 3. All subscales were significantly correlated (ps < 5 × 10−9) and exhibited acceptable internal reliability. In the MLMM, gender, age, and site were each found to be significant, respectively F (4, 976) = 11.18, p = 1.14 × 10−13, Wilk’s Λ = .93, partial η2 = .08; F (4, 976) = 1.80, p = 6.0 × 10−6, Wilk’s Λ = 0.85, partial η2 = .02; F (4, 976) = 4.37, p = .00009, Wilk’s Λ = .97, partial η2 = .03, and were thus included as covariates. Examination of Pearson correlations among the covariates and impulsive personality subscales indicated that individuals from the Athens site were more impulsive and, generally consistent with the literature, males had significantly higher positive urgency and motor impulsivity (Coskunpinar and Cyders, 2013), and younger individuals were more impulsive across four of the subscales (Steinberg et al., 2008) (see supplementary Table S1). Income was not significantly related to the impulsive personality subscales (F (4, 976) = 1.06, p = .38, Wilk’s Λ = 0.97, partial η2 = .01) and therefore was not covaried in all other analyses.
Table 3.
Pearson correlations among impulsive personality traits
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Attentional (α = .70) | --- | --- | --- | --- | --- | --- |
| 2. Motor (α = .63) | .378* | --- | --- | --- | --- | --- |
| 3. Non-planning (α = .68) | .413* | .474* | --- | --- | --- | --- |
| 4. Negative Urgency (α =.87) | .399* | .296* | .411* | --- | --- | --- |
| 5. Premeditation (α = .85) | .313* | .479* | .592* | .307* | --- | --- |
| 6. Perseverance (α = .84) | .440* | .174* | .494* | .357* | .362* | --- |
| 7. Positive Urgency (α = .93) | .332* | .280* | .344* | .594* | .323* | .275* |
Note:
p < 5 × 10−9.
3.2. A priori loci
Of the 14 a priori loci assessed, two loci (rs6313, rs6311) were significantly associated with impulsive personality in the multivariate analyses (nominal ps = .002, .003, respectively; see Table 4). These two associations survive even a stringent Bonferroni correction among the a priori loci (adjusted α = .004). Notably, rs6313 and rs6311 are essentially in total linkage disequilibrium (LD) and therefore offer largely redundant information (r2 = .996). In examining the individual subscales, these two loci were selectively significantly associated with the Motor (rs6313: B = −.101, SE = .046, p = .027; rs6311: B = −.098, SE = .046, p = .032) and Non-planning subscales (rs6313: B = −.122, SE = .046, p = .008; rs6311: B = −.119, SE = .046, p = .010). The T allele of rs6313 and the A allele of rs6311 were associated with lower impulsivity. The lower significance values (p = .002, .003) at the multivariate level as compared to the individual subscale level (ps .008–.03) attest to the value of utilizing this statistical approach when exploring multiple correlated phenotypes (Galesloot et al., 2014).
Table 4.
Associations between a priori loci and impulsive personality traits.
| Chr | Locus | Gene | Missing | Minor Allele | MAF | P |
|---|---|---|---|---|---|---|
| 3 | rs1732170 | GSK3β | 0 | T | .402 | .466 |
| 5 | rs6295 | HTR1A | 2 | C | .497 | .878 |
| 6 | rs13212041 | HTR1B | 34 | C | .216 | .914 |
| 6 | rs1799971 | OPRM1 | 0 | G | .127 | .957 |
| 11 | rs6265 | BDNF | 0 | T | .200 | .487 |
| 11 | rs1800497 | ANKK1 | 0 | A | .183 | .875 |
| 12 | rs2228570 | VDR | 0 | A | .414 | .475 |
| 13 | rs63131 | HTR2A | 0 | A | .419 | .002 |
| 13 | rs63111 | HTR2A | 7 | T | .418 | .003 |
| 14 | rs11624704 | NRXN3 | 0 | C | .150 | .754 |
| 22 | rs4680 | COMT | 0 | G | .482 | .780 |
| 5 | rs3814424c | LINC00461 | 15 | T | .170 | .229 |
| 8 | rs6981523n | XKR6 | 7 | C | .460 | .542 |
| 22 | rs9611519n | L3MBTL2/CHADL | 1 | T | .271 | .995 |
Note.
loci are in high LD (i.e., r2 = .996). Bolding of loci indicates significant effects were identified. Follow-up univariate analyses were only conducted for loci with significant multivariate effects.
previously associated with Conscientiousness and Neuroticism, respectively, in recent GWAS (Lo et al., 2016).
Given the differences in impulsivity by site, we conducted exploratory analyses testing a priori SNPs by site interactions in GEMMA and using a Bonferroni correction to minimize Type I errors. No significant interactions were present.
3.3. Genome-wide associations
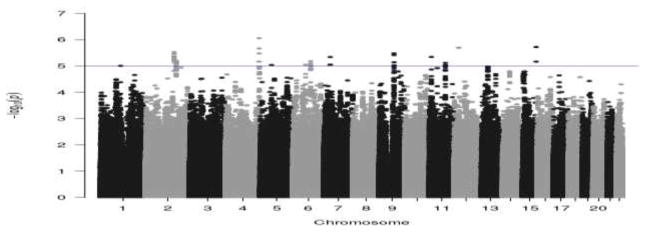
The genome-wide scan did not yield any significant associations at p < 5 × 10−8. The strongest association was rs13122329 in the STOX2 gene on chromosome 4 (p = 8.81E-7). The next two strongest associations were rs35721523 in an uncharacterized region (LOC101927263) in a non-coding RNA gene on chromosome 15 (p = 1.89E-6) and rs67068739 in the lamin domain tail containing 1 (LMNTD1) gene on chromosome 12 (p = 2.02E-6). The top 50 most significant hits are included in supplementary materials (Table S2). The results of the GWAS are depicted using both Manhattan plots (Figure S2) and Quantile-Quantile (Q-Q) plots (Figure S3). As can be seen in the Q-Q plot, the majority of markers fit null expectations and no markers suggest associations beyond chance.
4. Discussion
This study sought to assess genetic influences on impulsive personality traits, one of three major domains comprise the superordinate construct of impulsivity, to better understand heritable risk for addiction and other psychiatric disorders involving disinhibition. We examined the genetic basis of impulsive personality traits using a hierarchical approach to examine a priori loci and genome-wide variation. In terms of the a priori loci, we identified robust evidence for two previously identified candidate loci (rs6313 and rs6311) within HTR2a, but failed to support the remaining 12 loci. Specifically, the T allele of rs6313 and the A allele of rs6311 were associated with lower levels of impulsivity, with effects specific to the BIS-11 Motor and Non-planning subscales. The rs6311 finding is consistent with a study that found the GG genotype was associated with higher BIS-11 total scores in 135 individuals with alcohol dependence (Preuss et al., 2001), but is not consistent with other studies (Racine et al., 2009; Jakubczyk et al., 2012). These differences in findings may be attributable to smaller and different samples in the previous studies (e.g., females only, individuals with alcohol dependence).
One recent meta-analysis explored the relationships of these two loci with alcohol and drug abuse (Cao et al., 2014). Specifically, the meta-analysis found the T allele rs6313 was protective in studies of opioid and alcohol dependence/abuse and this result extended to combined analyses with the Study of Addiction: Genetics and Environment (SAGE) dataset. However, for rs6311, there was some evidence of the A allele being associated with alcohol dependence/abuse in Europeans, but this did not replicate in the combined analyses with the SAGE dataset. Cao et al.’s results are somewhat surprising because the T allele and A allele are in almost total LD, and therefore these minor alleles should have consistent effects, either promoting or protecting risk from drug abuse.
Although neither rs6313 nor rs6311 alter the encoded protein, recent work on the direct role of these SNPs on expression of the HTR2A gene has provided valuable clues into the biological mechanism of allelic variation. The C allele of rs6313 and G allele of rs6311 have been found to be associated with higher expression of 5′ UTR in HTR2A (Ruble et al., 2016; Smith et al., 2014, 2013). Greater expression of 5′ UTR is associated with greater translational efficiency and protein production, presumably leading to widespread higher 5-HT2A concentration (Smith et al., 2014, 2013). 5-HT2A interacts with numerous neurotransmitter systems, and injections of agonists or blockades in rats can have opposing effects on impulsivity, depending on the region the injection occurs (Hadamitzky et al., 2009; Robinson et al., 2008; Wischhof et al., 2011). The functionality of the A allele of rs6311 and T allele of rs6313 is presumably protective given their association with lower impulsivity in this study, but additional studies will be needed to clarify the relationship between these loci, gene function, and risk for impulsivity and addictive disorders.
Beyond these alleles, it was notable that the other a priori candidate SNPs tested were not replicated, which may be related to features of our subject sample, unidentified SNP by environment effects (e.g., childhood adversity; Carver et al., 2014; Dick et al., 2015), or the previous findings may be false-positives. The analyses of genome-wide loci yielded no statistically significant associations, perhaps because the present sample of 983 was underpowered for high-dimensional and genome-wide analysis of these traits. Nonetheless, given the rigorous phenotypic assessment, the loci with the highest associations still inform future analyses. Indeed, the top 50 loci are provided in the supplementary materials so that future investigations can prioritize these suggestive loci.
This study has several limitations that bear mentioning. The sample size was sufficient for strong tests of small effect sizes in a priori loci, but only to detect large effect sizes in the genome-wide tests. We recruited a low substance exposure sample to minimize possible confounding effects of drug use on impulsive personality traits, but this also limited the ability to generalize to studies that tested some of the a priori loci in individuals exhibiting problematic substance use. In addition, by recruiting a generally healthy sample, we may have excluded other impulsive subpopulations. An additional limitation is that we did not account for history of moderate to severe traumatic brain injury which, similar to heavy drug use, is an environmental exposure that may increase impulsive personality traits (e.g., Rochat et al., 2010; Wood and McHugh, 2013). However, this is mitigated somewhat by the low base rate of traumatic brain injury in the general population (Roozenbeek et al., 2013) and presumably even lower rate in this selected sample. Furthermore, this study was not designed to test the overlapping heritability between addictive disorders and impulsive personality traits. It will be important for future investigations to test overlapping heritability of impulsive personality traits and addictive disorders (and other disorders of disinhibition) as well as verify the findings from this study in well-powered investigations of healthy samples and samples matched in psychopathology to previous investigations. Our finding that BIS-11 Motor and Non-planning subscales are associated with rs6313 and rs6311 suggest that these SNPs may contribute to intermediate mechanisms (i.e., via impulsive personality traits) that contribute to addictive disorders or other conditions associated with impulsivity, but these links could not be tested directly in this study. Finally, although it was beyond the scope of this study to explore the genetics of other domains of impulsivity (i.e., response inhibition and decision making), these domains are no less relevant to addiction and other disorders of disinhibition (Amlung et al., 2016; Jackson and MacKillop, 2016; Smith et al., 2014) and thus should be explored as well.
Nonetheless, this study makes novel contributions to our understanding of genetic influences on impulsive personality traits, particularly in the support for previously published studies implicating SNPs in HTR2A. Future studies with larger sample sizes will be necessary to reveal other associations reliably, but, considering the robust evidence that impulsive personality traits are both associated with a variety of psychiatric conditions and are heritable (Bevilacqua and Goldman, 2013), continued inquiry into its genetic determinants remains an important goal.
Supplementary Material
Figure 1.
Manhattan plot of genome-wide association for impulsive personality traits multivariate regression models with adjustment for sex, age, and site. Significance values were – log10 transformed in order to display the smaller p-values as larger in the figure. The Manhattan plot displays level of significance for each SNP, organized by chromosomal position from chromosomes 1–22. The blue line indicates suggestive significance (10−5). No SNPs achieve genome-wide significance (p < 5 × 10−8).
Highlights.
A multivariate genetic analysis is applied to impulsive personality traits (IPTs).
Significant loci within the HTR2A gene are identified.
Doubt is cast on many previously identified candidate loci for IPTs.
The GWAS results highlight suggestive candidates for future investigation.
Acknowledgments
We are very grateful to Dr. Steven Beach and Dr. Lawrence Sweet for their feedback with early drafts of the manuscript. We are also very grateful for the contributions of the undergraduate volunteers for their role in data collection and entry.
Funding
Funding for this study was provided by NIH Grant R01DA032015. JM is the holder of the Peter Boris Chair in Addictions Research, which partially supported his role.
Footnotes
Conflict of interest
The authors do not have any actual or potential conflict of interest to disclose.
Disclaimer
The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Contributors
HDW, AAP, and JM were responsible for the study concept; JM, HDW, and AAP were responsible for study design and protocol; JCG and JW provided additional refinement to the study design and protocol; JCG and JW contributed to data collection; JCG and JJ conducted data analysis; JCG, JM, HDW, AAP, and JW contributed to data interpretation; JG drafted the manuscript; JM, HDW, AAP, and JW provided critical revision for important intellectual content. KMH conducted imputation. AAP, KMH, and JJ consulted on quality control of genetic data. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction. 2016;112:51–62. doi: 10.1111/add.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- Benko A, Lazary J, Molnar E, Gonda X, Tothfalusi L, Pap D, Mirnics Z, Kurimay T, Chase D, Juhasz G, Anderson IM, Deakin JFW, Bagdy G. Significant association between the C(-1019)G functional polymorphism of the HTR1A gene and impulsivity. Am J Med Genet Part B, Neuropsychiatr Genet. 2010;153B:592–599. doi: 10.1002/ajmg.b.31025. [DOI] [PubMed] [Google Scholar]
- Berg JM, Latzman RD, Bliwise NG, Lilienfeld SO. Parsing the heterogeneity of impulsivity: A meta-analytic review of the behavioral implications of the UPPS for psychopathology. Psychol Assess. 2015;27:1129–1146. doi: 10.1037/pas0000111. [DOI] [PubMed] [Google Scholar]
- Berman AH, Bergman H, Palmstierna T, Schlyter F. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res. 2005;11:22–31. doi: 10.1159/000081413. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Goldman D. Genetics of impulsive behaviour. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120380. doi: 10.1098/rstb.2012.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Liu X, Han S, Zhang CK, Liu Z, Li D. Association of the HTR2A gene with alcohol and heroin abuse. Hum Genet. 2014;133:357–365. doi: 10.1007/s00439-013-1388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J, Kim Y, Nam JY. Serotonin transporter polymorphism interacts with childhood adversity to predict aspects of impulsivity. Psychol Sci. 2011;22:589–595. doi: 10.1177/0956797611404085. [DOI] [PubMed] [Google Scholar]
- Carver CS, LeMoult J, Johnson SL, Joormann J. Gene effects and G x E interactions in the differential prediction of three aspects of impulsiveness. Soc Psychol Personal Sci. 2014;5:730–739. doi: 10.1177/1948550614527116. [DOI] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015:4. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester DS, DeWall CN, Derefinko KJ, Estus S, Peters JR, Lynam DR, Jiang Y. Monoamine oxidase A (MAOA) genotype predicts greater aggression through impulsive reactivity to negative affect. Behav Brain Res. 2015;283:97–101. doi: 10.1016/j.bbr.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- Coskunpinar A, Cyders MA. Impulsivity and substance-related attentional bias: a meta-analytic review. Drug Alcohol Depend. 2013;133:1–14. doi: 10.1016/j.drugalcdep.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Coskunpinar A, Dir AL, Cyders MA. Multidimensionality in impulsivity and alcohol use: a meta-analysis using the UPPS model of impulsivity. Alcohol Clin Exp Res. 2013;37:1441–50. doi: 10.1111/acer.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C. Integration of impulsivity and positive mood to predict risky behavior: Development and validation of a measure of positive urgency. Psychol Assess. 2007;19:107–118. doi: 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, Hewitt JK, Kendler KS, Sher KJ. Candidate gene–environment interaction research: Reflections and recommendations. Perspect Psychol Sci. 2015;10:37–59. doi: 10.1177/1745691614556682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Latendresse S, Porjesz B, Schuckit M, Rangaswamy M, Hesselbrock V, Edenberg H, Nurnberger J, Agrawal A, Bierut L, Wang J, Bucholz K, Kuperman S, Kramer J. How phenotype and developmental stage affect the genes we find: GABRA2 and impulsivity. Twin Res Hum Genet. 2013;16:661–669. doi: 10.1017/thg.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, Derom CA, Thiery EW, Grimmer MA, Martin NG, Boomsma DI. Heritability of borderline personality disorder features is similar across three countries. Psychol Med. 2008;38:1219–1229. doi: 10.1017/S0033291707002024. [DOI] [PubMed] [Google Scholar]
- Doran N, Trim RS. The prospective effects of impulsivity on alcohol and tobacco use in a college sample. J Psychoactive Drugs. 2013;45:379–85. doi: 10.1080/02791072.2013.844380. [DOI] [PubMed] [Google Scholar]
- Eisenberg DTA, Campbell B, Mackillop J, Lum JK, Wilson DS. Season of birth and dopamine receptor gene associations with impulsivity, sensation seeking and reproductive behaviors. PLoS One. 2007;2:e1216. doi: 10.1371/journal.pone.0001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–80. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galesloot TE, van Steen K, Kiemeney LALM, Janss LL, Vermeulen SH. A comparison of multivariate genome-wide association methods. PLoS One. 2014;9:e95923. doi: 10.1371/journal.pone.0095923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gustavson DE, Miyake A, Hewitt JK, Friedman NP. Genetic relations among procrastination, impulsivity, and goal-management ability: Implications for the evolutionary origin of procrastination. Psychol Sci. 2014;25:1178–1188. doi: 10.1177/0956797614526260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky M, Feja M, Becker T, Koch M. Effects of acute systemic administration of serotonin2A/C receptor ligands in a delay-based decision-making task in rats. Behav Pharmacol. 2009;20:415–423. doi: 10.1097/FBP.0b013e3283305e11. [DOI] [PubMed] [Google Scholar]
- Hart AB, de Wit H, Palmer AA. Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology. 2013;38:802–816. doi: 10.1038/npp.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population Stratification in the Candidate Gene Study: Fatal Threat or Red Herring? Psychol Bull. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Jackson JN, MacKillop J. Attention-deficit/hyperactivity disorder and monetary delay discounting: a meta-analysis of case-control studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:316–325. doi: 10.1016/j.bpsc.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubczyk A, Wrzosek M, Lukaszkiewicz J, Sadowska-Mazuryk J, Matsumoto H, Sliwerska E, Glass J, Burmeister M, Brower KJ, Wojnar M. The CC genotype in HTR2A T102C polymorphism is associated with behavioral impulsivity in alcohol-dependent patients. J Psychiatr Res. 2012;46:44–49. doi: 10.1016/j.jpsychires.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen JRM, Rydkjaer J, Fagerlund B, Pagsberg AK, Jespersen Rav F, Glenthøj BY, Oranje B. Overlapping and disease specific trait, response, and reflection impulsivity in adolescents with first-episode schizophrenia spectrum disorders or attention-deficit/hyperactivity disorder. Psychol Med. 2017:1–13. doi: 10.1017/S0033291717001921. [DOI] [PubMed] [Google Scholar]
- Jiménez E, Arias B, Mitjans M, Goikolea JM, Roda E, Ruíz V, Pérez A, Sáiz PA, Paz García-Portilla M, Burón P, Bobes J, Vieta E, Benabarre A. Association between GSK3β gene and increased impulsivity in bipolar disorder. Eur Neuropsychopharmacol. 2014;24:510–518. doi: 10.1016/j.euroneuro.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Laas K, Eensoo D, Paaver M, Lesch K-P, Reif A, Harro J. Further evidence for the association of the NPSR1 gene A/T polymorphism (Asn107Ile) with impulsivity and hyperactivity. J Psychopharmacol. 2015;29:878–883. doi: 10.1177/0269881115573803. [DOI] [PubMed] [Google Scholar]
- Limosin F, Loze JY, Dubertret C, Gouya L, Ades J, Rouillon F, Gorwood P. Impulsiveness as the intermediate link between the dopamine receptor D2 gene and alcohol dependence. Psychiatr Genet. 2003;13:127–129. doi: 10.1097/01.ypg.0000066963.66429.00. [DOI] [PubMed] [Google Scholar]
- Lo M-T, Hinds DA, Tung JY, Franz C, Fan C-C, Wang Y, Smeland OB, Schork A, Holland D, Kauppi K, Sanyal N, Escott-Price V, Smith DJ, O’Donovan M, Stefansson H, Bjornsdottir G, Thorgeirsson TE, Stefansson K, McEvoy LK, Dale AM, Andreassen OA, Chen C-H. Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nat Genet. 2016;49:152–156. doi: 10.1038/ng.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Munafò M. An intermediate phenotype approach to addiction genetics. In: MacKillop J, Munafò M, editors. Genetic Influences on Addiction: An Intermediate Phenotype Approach. MIT Press; Cambridge: 2013. pp. 1–19. [Google Scholar]
- MacKillop J, Weafer J, Gray J, Oshri A, Palmer AA, de Wit H. The latent structure of impulsivity: impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacol. 2016;233:3361–3370. doi: 10.1007/s00213-016-4372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Clark TG, Flint J. Assessing publication bias in genetic association studies: evidence from a recent meta-analysis. Psychiatry Res. 2004;129:39–44. doi: 10.1016/j.psychres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Németh N, Kovács-Nagy R, Székely A, Sasvári-Székely M, Rónai Z. Association of impulsivity and polymorphic microRNA-641 target sites in the SNAP-25 gene. PLoS One. 2013;8:e84207. doi: 10.1371/journal.pone.0084207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv S, Tuvblad C, Raine A, Wang P, Baker LA. Heritability and longitudinal stability of impulsivity in adolescence. Behav Genet. 2012;42:378–392. doi: 10.1007/s10519-011-9518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaver M, Nordquist N, Parik J, Harro M, Oreland L, Harro J. Platelet MAO activity and the 5-HTT gene promoter polymorphism are associated with impulsivity and cognitive style in visual information processing. Psychopharmacology (Berl) 2007;194:545–554. doi: 10.1007/s00213-007-0867-z. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35:2414–2426. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pe’er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008;32:381–385. doi: 10.1002/gepi.20303. [DOI] [PubMed] [Google Scholar]
- Pfeifer P, Sariyar M, Eggermann T, Zerres K, Vernaleken I, Tüscher O, Fehr C. Alcohol consumption in healthy OPRM1 G allele carriers and its association with impulsive behavior. Alcohol Alcohol. 2015 doi: 10.1093/alcalc/agv019. agv019–. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Koller G, Bondy B, Bahlmann M, Soyka M. Impulsive traits and 5- HT2A receptor promoter polymorphism in alcohol dependents: Possible association but no influence of personality disorders. Neuropsychobiology. 2001;3:186–191. doi: 10.1159/000054888. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Harden KP. Differential changes in impulsivity and sensation seeking and the escalation of substance use from adolescence to early adulthood. Dev Psychopathol. 2013;25:223–239. doi: 10.1017/S0954579412000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Stappenbeck CA, Fromme K. Collegiate heavy drinking prospectively predicts change in sensation seeking and impulsivity. J Abnorm Psychol. 2011;120:543–556. doi: 10.1037/a0023159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Culbert KM, Larson CL, Klump KL. The possible influence of impulsivity and dietary restraint on associations between serotonin genes and binge eating. J Psychiatr Res. 2009;43:1278–1286. doi: 10.1016/j.jpsychires.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Pers Individ Dif. 2006;40:305–315. [Google Scholar]
- Robinson ESJ, Dalley JW, Theobald DEH, Glennon JC, Pezze MA, Murphy ER, Robbins TW. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-Choice Serial Reaction Time Task. Neuropsychopharmacology. 2008;33:2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- Rochat L, Beni C, Billieux J, Azouvi P, Annoni J-M, Van der Linden M. Assessment of impulsivity after moderate to severe traumatic brain injury. Neuropsychol Rehabil. 2010;20:778–797. doi: 10.1080/09602011.2010.495245. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Müller U, Clark L, Sahakian BJ. The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. Int J Neuropsychopharmacol. 2007;10:449–461. doi: 10.1017/S146114570600705X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozenbeek B, Maas AIR, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9:231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- Ruble CL, Smith RM, Calley J, Munsie L, Airey DC, Gao Y, Shin JH, Hyde TM, Straub RE, Weinberger DR, Nisenbaum LK. Genomic structure and expression of the human serotonin 2A receptor gene (HTR2A) locus: identification of novel HTR2A and antisense (HTR2A-AS1) exons. BMC Genet. 2016;17:16. doi: 10.1186/s12863-015-0325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakado K, Sakado M, Muratake T, Mundt C, Someya T. A psychometrically derived impulsive trait related to a polymorphism in the serotonin transporter gene-linked polymorphic region (5-HTTLPR) in a Japanese nonclinical population: assessment by the Barratt impulsiveness scale (BIS) Am J Med Genet B Neuropsychiatr Genet. 2003;121B:71–75. doi: 10.1002/ajmg.b.20063. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schilling C, Kuhn S, Sander T, Gallinat J. Association between dopamine D4 receptor genotype and trait impulsiveness. Psychiatr Genet. 2014;24:82. doi: 10.1097/YPG.0000000000000005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. A brief history of research on the genetics of alcohol and other drug use disorders. J Stud Alcohol Drugs Suppl. 2014;75(Suppl 1):59–67. doi: 10.15288/jsads.2014.s17.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroczynski AD, Bergeman C, Coccaro EF. Etiology of the impulsivity/aggression relationship: Genes or environment? Psychiatry Res. 1999;86:41–57. doi: 10.1016/s0165-1781(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Smith RM, Banks W, Hansen E, Sadee W, Herman GE. Family-based clinical associations and functional characterization of the serotonin 2A receptor gene (HTR2A) in Autism Spectrum Disorder. Autism Res. 2014;7:459–467. doi: 10.1002/aur.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Smith RM, Papp AC, Webb A, Ruble CL, Munsie LM, Nisenbaum LK, Kleinman JE, Lipska BK, Sadee W. Multiple regulatory variants modulate expression of 5-hydroxytryptamine 2A receptors in human cortex. Biol Psychiatry. 2013;73:546–554. doi: 10.1016/j.biopsych.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro-De-Souza MG, Stanford MS, Bio DS, Machado-Vieira R, Moreno RA. Association of the COMT Met158 allele with trait impulsivity in healthy young adults. Mol Med Rep. 2013;7:1067–1072. doi: 10.3892/mmr.2013.1336. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers Individ Dif. 2009;47:385–395. doi: 10.1016/j.paid.2009.04.008. [DOI] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Dev Psychol. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Lehmann MK, Christ CC, Hersrud SL, Davies GE. Associations among types of impulsivity, substance use problems and Neurexin-3 polymorphisms. Drug Alcohol Depend. 2011;119:e31–e38. doi: 10.1016/j.drugalcdep.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Tao J, Zhang J, Xie Y, Sun Y, Li L, Xu K, Han B, Lu Y, Sun H, Wei Y, Wang Y, Zhang Y, Zou S, Wu W, Zhang J, Zhang X, He J. An association between BDNF Val66Met polymorphism and impulsivity in methamphetamine abusers. Neurosci Lett. 2014;582:16–20. doi: 10.1016/j.neulet.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Varga G, Szekely A, Antal P, Sarkozy P, Nemoda Z, Demetrovics Z, Sasvari-Szekely M. Additive effects of serotonergic and dopaminergic polymorphisms on trait impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:281–288. doi: 10.1002/ajmg.b.32025. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30:669–689. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- Wischhof L, Hollensteiner KJ, Koch M. Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav Pharmacol. 2011;22:805–813. doi: 10.1097/FBP.0b013e32834d6279. [DOI] [PubMed] [Google Scholar]
- Wood RL, McHugh L. Decision Making after Traumatic Brain Injury: A Temporal Discounting Paradigm. J Int Neuropsychol Soc. 2013;19:181–188. doi: 10.1017/S135561771200118X. [DOI] [PubMed] [Google Scholar]
- Wrzosek M, Jakubczyk A, Wrzosek M, Kaleta B, Łukaszkiewicz J, Matsumoto H, Brower K, Nowicka G, Wojnar M. Association between Fok I vitamin D receptor gene (VDR) polymorphism and impulsivity in alcohol-dependent patients. Mol Biol Rep. 2014;41:7223–7228. doi: 10.1007/s11033-014-3607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J of Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat Genet. 2012;44:821–824. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.