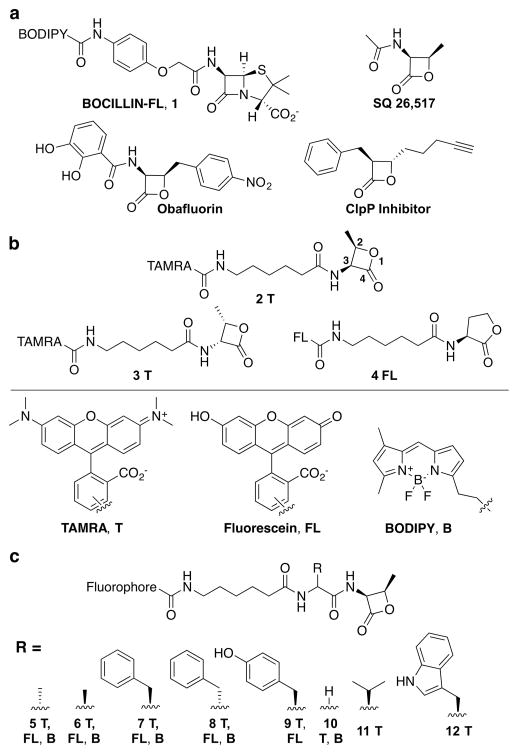
Figure 2.
β-lactone-based probe library. (a) Structures of BOCILLIN-FL and three lactone-containing molecules with antibacterial activity. (b) Lactone probes bearing one of three fluorophores: 5(6)-carboxytetramethylrhodamine (TAMRA, T), 5(6)-fl uorescein (FL), and boron-dipyrromethene (BODIPY, B). These probes include a stereochemical mimic of biologically active β-lactams (2R,3S-β-lactone; 2T, FL, B), the opposite isomer (2S,3R-β-lactone; 3T, B), and a five-membered ring δ-lactone (4FL). (c) Amino acid components were incorporated to yield probes with functional groups found in the natural peptidoglycan substrate, D-Ala (5), and the opposite stereoisomer, L-Ala (6), or in β-lactam antibiotics, L-Phe (7), D-Phe (8), and L-Tyr (9), and Gly to assess the importance of the side chain (10). Two additional hydrophobic amino acids were also incorporated, L-Val (11) and L-Trp (12).