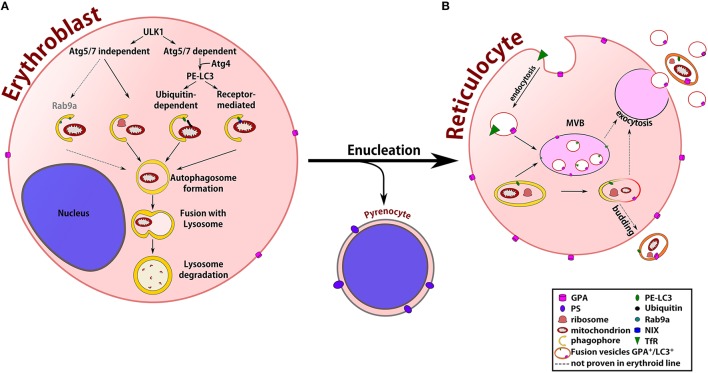
Figure 1.
Terminal maturation of erythroblasts. (A) At the erythroblast stage, two Ulk1-mediated autophagic pathways are activated to allow organelle clearance: the Atg5/7-dependent pathway with the proteolytic Atg4-dependent activation of MAPLC3, microtubule-associated protein 1 light channel 3 (LC3) and the Atg5/7-independent pathway, which is not related to the LC3 protein. LC3 activation allows its insertion into the phagophore membrane, starting the engulfment of organelles through the recognition of an ubiquitin signal or by the direct binding of specialized receptors at the organelle membrane. In non-erythroid cells, Rab9a is important for the formation of the phagophore during the Atg5/7-independent autophagic pathway. After the formation of the autophagosome, its fusion with the lysosome permits the degradation of organelles by hydrolytic enzymes. The enucleation process gives rise to the pyrenocyte and the reticulocyte, which still contains some organelles that must be eliminated for the final maturation into erythrocyte. (B) During this stage, unwanted membrane proteins, such as transferrin receptor (TfR), are internalized by endocytosis and expelled by exocytosis from multi-vesicular body structures. Glycophorin A (GPA)/LC3 double-positive vesicles containing organelle remnants are also found in reticulocytes, suggesting cooperation between the endocytosis (GPA+) and autophagy (LC3+) pathways to eliminate organelles. How autophagosomes interact with multivesicular bodies (MVBs) following the same pathway of membrane protein recycling or budding directly from the plasma membrane after fusion with endocytic vesicles, however, remains unknown.