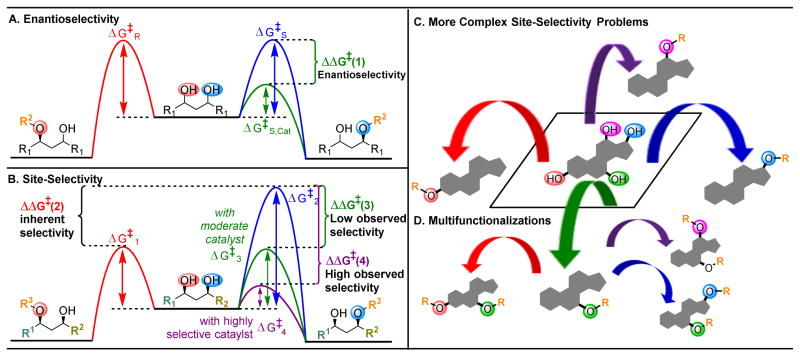
Figure 2.
(A) Energy profiles of a desymmetrization reaction, where both potentially reacting alcohols are chemically equivalent. A catalyst that can selectively lower the activation barrier (
 pathway,
pathway,
 ) will result in high enantioselectivities,4
(B) Energy profiles of a site-selective transformation, where reactive groups are nonequivalent. Depending on the inherent energy profiles of the functional groups (
) will result in high enantioselectivities,4
(B) Energy profiles of a site-selective transformation, where reactive groups are nonequivalent. Depending on the inherent energy profiles of the functional groups (
 versus
versus
 pathways,
pathways,
 ), catalytic reduction of an energy barrier may not result in high observed selectivities (
), catalytic reduction of an energy barrier may not result in high observed selectivities (
 pathway,
pathway,
 ). The achievement of highly selective functionalizations may require substantially more selective catalysts (
). The achievement of highly selective functionalizations may require substantially more selective catalysts (
 pathway,
pathway,
 ). (C) This problem is compounded by the addition of more reactive groups and (D) the ability for substrates to undergo multiple derivatization events.
). (C) This problem is compounded by the addition of more reactive groups and (D) the ability for substrates to undergo multiple derivatization events.