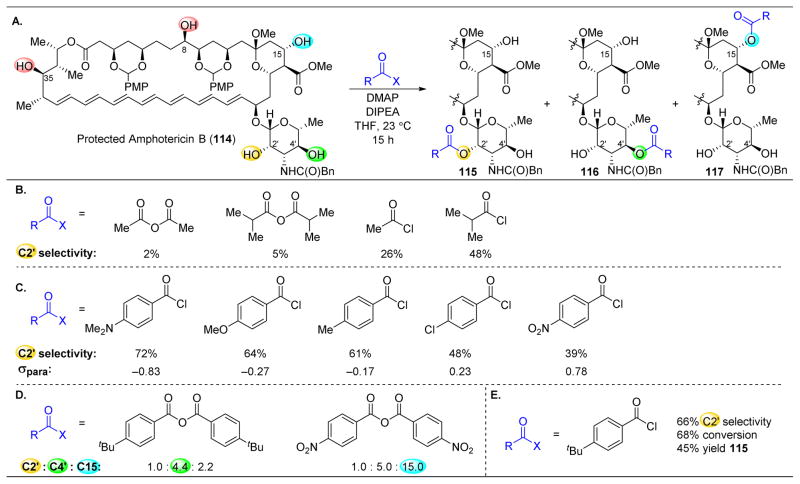
Figure 21.
(A) Site-selective acylation of protected amphotericin B (114). (B) The more sterically hindered the acyl transfer reagent, the higher the C2′-selectivity. (C) The more electron-rich the benzoyl chloride, the less reactive the reagent is and more C2′ selectivity is observed. (D) Tuning of acid anhydrides results in two additional site-selective reactions. (E) Optimized acylating agent. p-tertbutylbenzoyl chloride.150