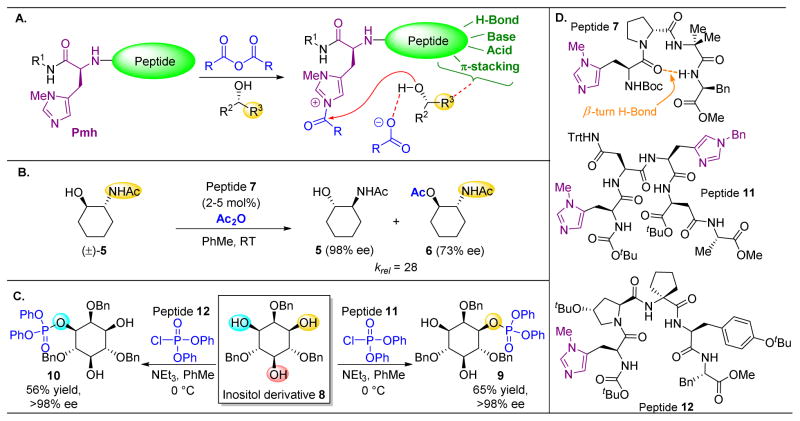
Figure 4.
(A) Pmh-catalyzed acyl transfer. The imidazole (or other N-heterocycles) serves as a nucleophilic catalyst, decomposing the acid anhydride and delivering the acyl group to a substrate hydroxyl. Other functionality on the peptide can bind to the substrate and enforce selectivity. (B) The acetamide of 5 serves as a directing group for peptide 7, resulting in high levels of selectivity for this kinetic resolution by acylation.39 (C) Peptide-based phosphorylation can be accomplished on more complex substrates, such as 8. (D) Peptides utilized in this figure.48