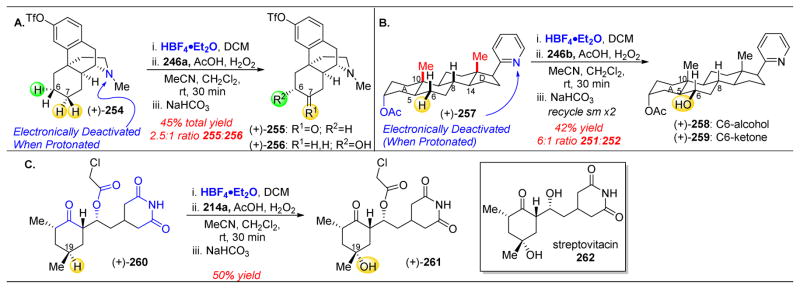
Figure 43.
(A) Oxidation of dextrometliorphan derivative (+)-254 revealed preferences for oxidation of the C6–H and one of the C7–H bonds, the most distal groups from the protonated piperazine ring. (B) Oxidation of abiraterone acetate analogue (+)-257 shows preference for the C6–H bond, which is distant from the EWGs on the A and D rings. This is aided by the strain release that is afforded in the planarization of C6 from the axial methyl group at C10. The catalyst is also able to select for C6–H against tertiary C–H bonds at C5, C8, and C14. (C) Cycloheximine derivative (+)-260 is oxidized at C19–H, which is furthest away from the multiple EWGs of the molecule.234