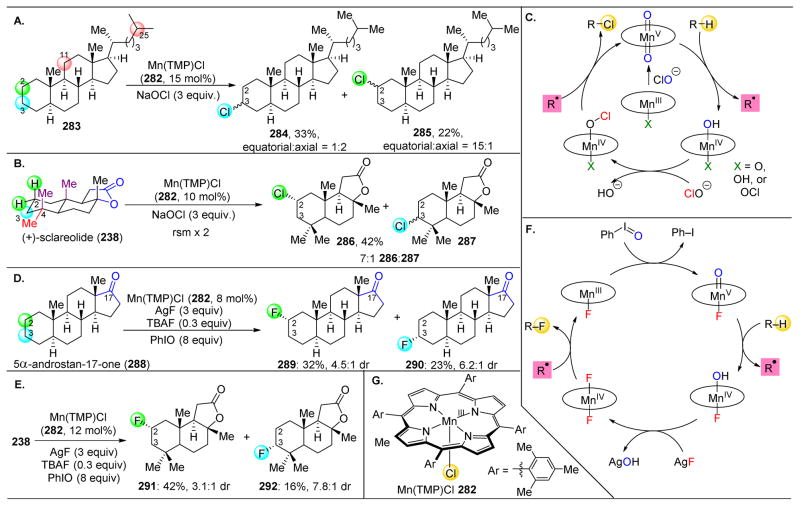
Figure 46.
(A) Chlorination of 5α-cholestane (283) with Mn-porphyrin 282 results in the selective formation of 284 and 285. (B) Chlorination of sclareolide (238) results in preferential formation of the equatorial C2-chlorination product (286). (C) Proposed mechanism for the Mn-porphyrin catalyzed chlorinations. (D) Fluorination of 5α-androstan-17-one (288) results in preferential formation of 289 and 290. (E) Fluorination of sclareolide (238) with Mn-porphyrin 282 results in the selective formation of 291 and 292. (F) Proposed mechanism for the Mn-porphyrin catalyzed fluorination. (G) Structure of Mn(TMP)CI 284. Abrev. TMP: tetramesityporphyrin.242,243