Figure 1.
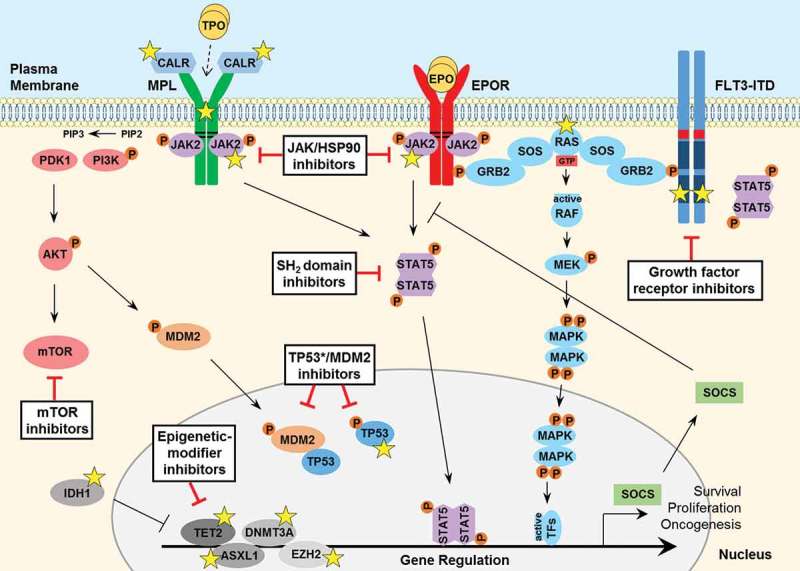
Signaling pathways involved in the pathogenesis of MPNs and secondary AML. JAK2 binds to the cytosolic juxta-membrane region of dimeric cytokine receptors such as MPL (TPOR) and EPOR, via the BOX1 and BOX2 receptor motifs (black lines). JAK2 activation (via receptor-ligand binding or gain-of-function mutation such as JAK2 V617F) promotes various downstream signaling pathways, via STAT5, including RAS-MAPK and PI3K-AKT. These pathways facilitate oncogenic gene transcription and promote cancer cell survival, proliferation or migration. The expression of negative regulators such as the SOCS proteins are induced by the JAK-STAT pathway, however they are not sufficient to block hyperactive JAK-STAT signaling and cannot bind JAK2 V617F. The FLT3-ITD mutant growth factor receptor commonly found in AML patients signals independently of ligand-binding, as a result of the internal tandem duplication (ITD) found within the juxta-membrane domain (red box) and point mutations that occur within the kinase domain (most frequently at D835; dark blue box) of the FLT3 receptor. FLT3-ITD hyperactivation promotes RAS-MAPK, PI3K-AKT as well as STAT5 signaling. A number of important somatic mutations have been reported in various oncogenes and tumor suppressor proteins within these pathways (yellow stars), where such mutations are known to contribute to disease initiation and progression. For further details on these mutations, see Table 1. Mutated calreticulin (CALR), frequently found in MPN patients, interacts with the extracellular portion of the MPL receptor at the Endoplasmic Reticulum-Golgi apparatus and also at the cell surface, promoting direct dimerization, activation of JAK2 and downstream signaling, independently of TPO binding (which is required for normal MPL signaling, indicated by a dashed arrow). Loss-of-function mutations in the critical tumor suppressor protein TP53 are also reported generally in MPN patients that progress to secondary AML. Furthermore, various epigenetic-modifier proteins are found to be mutated in MPN patients, including isocitrate dehydrogenase 1 (IDH1), methylcytosine dioxygenase TET2, DNA methyltransferase 3A (DNMT3A), Polycomb group protein ASXL1 and the histone methyltransferase protein of polycomb repressive complex 2 (PRC2) EZH2. Promising therapeutic agents to target these key proteins/pathways in MPN/AML have been developed and are summarized here (black boxes). TPO, thrombopoietin; EPO, erythropoietin; GTP, guanosine triphosphate; TF, transcription factor.
