Table 3.
Compounds which exhibit a measurable AmpG-specific activity index.
| entry | ID | structure | analysis |
|---|---|---|---|
| 1 | SR-01000076250-2 |
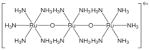
|
Inorganic dye: poor drug-likeness and poorly-suited for follow-up |
| 2 | SR-05000002238-3 |
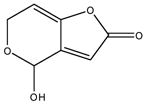
|
Patulin: natural product mycotoxin produced by a variety of molds. In ring-opened form it is chemically reactive, forming Schiff bases with biological amines. |
| 3 | SR-01000320782-1 |
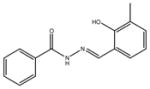
|
A synthetic compound: phenolic hydrazones are structure alerts and thus modifications to the structure in probe development would be desirable |
| 4 | SR-05000002074-1 |
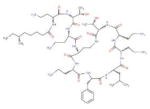
|
Natural product Polymyxin B, primarily used for resistant gram-negative infections. It is derived from the bacterium Bacillus polymyxa. |
| 5 | SR-01000226538-1 |
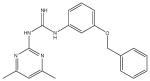
|
A synthetic compound in the aryl guanidine class. No stability or reactivity concerns. |
| 6 | SR-01000115944-1 |
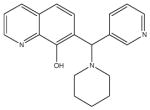
|
A synthetic compound in the 7-aminomethyl 8-hydroxyquinoline class. These are known as “Betti bases” and may be chemical unstable to retro-Michael addition. More chemically stable analogs would be preferred. |
| 7 | SR-01000115950-1 |
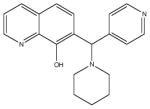
|
A structural isomer also in the 7-aminomethyl 8-hydroxyquinoline class. More chemically stable analogs would be preferred. |
| 8 | SR-01000520919-1 |
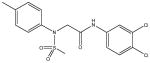
|
A synthetic compound in the N-aryl sulfonamide class. No stability or reactivity concerns. |
