Abstract
Background
The principal goal of this study was to determine the importance of high-sensitivity troponin T (hs-TnT) and creatine kinase MB isoenzyme (CK-MB) in predicting cardiovascular events in asymptomatic end-stage renal disease (ESRD) patients.
Methods
This study included 110 participants; 54 ESRD patients undergoing hemodialysis and 56 healthy control participants. Biochemical parameters and cardiac markers were estimated. Comparative utilities were assessed through logistic regression and receiver operating characteristic (ROC) analyses.
Results
We found that 96.3% of ESRD patients had an elevated level of hs-TnT (mean, 0.049 ± 0.0324 μg/L) compared to healthy participants. Among patients with ESRD, hs-TnT showed significant correlations with the low-density lipoprotein cholesterol/high-density lipoprotein cholesterol (HDL-C) (P = 0.042, r = 0.278) and total cholesterol/HDL-C (P = 0.044, r = 0.276) ratios. CK-MB (odds ratio [OR], 1.138; P = 0.04) and hs-TnT (OR, 2.153; P = 0.017) predicted cardiovascular events on logistic regression analysis, and the prediction was improved by the model that combined two cardiac markers. The diagnostic performance of hs-TnT and CK-MB alone and the combination of the two biomarkers was assessed by the area under the ROC curve (AUC). The highest AUC was produced by the combination of hs-TnT and CK-MB markers (0.920) compared to hs-TnT or CK-MB alone.
Conclusion
In asymptomatic patients with ESRD, hs-TnT appeared to be an important predictor for cardiovascular mortality, and its diagnostic accuracy improved with CK-MB. This study provides new insights into the predictive value of multiple biomarkers for identifying cardiovascular events in ESRD patients on hemodialysis.
Keywords: Cardiovascular diseases, Creatine kinase MB isoenzyme, End-stage renal disease, High-sensitivity troponin T
Introduction
In the United States, the incidence of end-stage renal disease (ESRD) is expected to increase by 29% to a prevalence of 47% by the end of 2020 [1]. Patients with ESRD have a considerably higher rate of cardiovascular disease (CVD) than the general population [2]. More than half of those with ESRD who are treated with chronic hemodialysis die from CVDs [3]. Paraskevas et al [4] reported that, in ESRD patients on hemodialysis, the mortality rate from CVD is about 20 times higher than that of age-matched individuals in the general population. This high cardiovascular burden supports the need for optimal cardiovascular risk-management in ESRD patients. In a very recent study, Collins and co-workers found that CVD is the largest cause of death, accounting for approximately 40% of the mortality of ESRD patients [5]. This excess cardiovascular morbidity and mortality of patients with ESRD could be contributed to many pathological processes, which might be associated with specific changes in the levels of ‘cardiac biomarkers’ [6]. Therefore, the identification of possible CVD risk factors associated with ESRD has become an important focus area for researchers.
Hence, a novel strategy for management of CVD in patients with ESRD on hemodialysis is the use of biochemical markers to facilitate early detection of cardiovascular abnormalities to allow better or effective therapy to be implemented. Troponin T and creatine kinase MB isoenzyme (CK-MB) are among the best studied of these CVD markers. However, controversy remains regarding the superiority of such results and the subsequent clinical application of these biomarkers, particularly when they are abnormal in patients with ESRD.
Although several studies have estimated a positive association of cardiac troponin T, CK-MB, and ESRD, there has been considerable reluctance to use the 99th percentile of the upper reference limit (URL) value. Many studies have not reported which generation of assay was used or the details of its cutoff point, including the manufacturer-reported 99th percentile threshold. As a result, the interpretation of increases in these values in hemodialysis patients with asymptomatic CVD is not reliable. In this context, the aim of this case-control study was to explore the prognostic value of high-sensitivity troponin T (hs-TnT) and CK-MB in the early detection of asymptomatic CVD in ESRD patients on maintenance hemodialysis, taking into account the 99th percentile URL values. Data that compare the association of both cardiac-specific bio-markers with each other and/or with CVD risks in ESRD are limited. Therefore, the present study also aimed to investigate whether both cardiac biomarkers are comparable in their ability to predict CVD risks using generations of the assays with sufficient sensitivity and specificity to detect the “abnormal” concentrations in clinically asymptomatic ESRD patients on hemodialysis and other patient populations.
Methods
Study participants
The current case-control study was conducted between November 2015 and November 2016, in 60 patients with ESRD undergoing hemodialysis in the Kidney Dialysis Center at AL-Thawra General Hospital in Ibb province of Yemen. The inclusion criteria for patients were as follows: patients with ESRD of both genders, aged 20 to 60 years, on regular hemodialysis for more than five months, and never received a renal transplant. Patients with clinical signs of heart failure, known muscle disease, liver disease, a functioning kidney transplant, severe anemia, malignancy, active inflammation, or those patients who performed resistance exercise training or were smokers were excluded from the study. The data were collected via direct interviews with patients during dialysis.
For comparison, 60 apparently healthy individuals who volunteer as blood donors at the Blood Bank Department (AL-Thawra General Hospital, Ibb province, Yemen) were recruited as controls. None of the healthy individuals developed any major illnesses during the study, had a history of renal disease, or were on any medication. Healthy participants were matched to the ESRD patients for gender and age and had to meet the exclusion criteria. Each participant included in the study agreed to attend the interview and complete a questionnaire. Patients and healthy individuals were given a full explanation about the purpose of the study and assured that their information would remain confidential during the study period and afterward. Blood pressure was measured by trained personnel according to the recommendations of the American Heart Association [7]. Hypertension is defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or current use of antihypertensive drugs in accordance with the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [8].
Anthropometric measurements included height to the nearest centimeter without shoes and weight to the nearest 0.1 kg in light clothing. Body mass index (BMI) was calculated by dividing weight (in kilograms) by height (in meters squared). BMI was classified into two categories according to the National Kidney Foundation’s cutoff points (< 23.8 and ≥ 23.8 kg/m2) [9]. An abnormal lipid profile was defined according to the Third Report of the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) [10]. Dyslipidemia was classified as the presence of any of the following: total cholesterol (TC) ≥ 200 mg/dL, low-density lipoprotein cholesterol (LDL-C) ≥160 mg/dL, high-density lipoprotein (HDL)-cholesterol < 40 mg/dL, triglycerides (TGs) ≥ 200 mg/dL, or the use of a statin drug. Blood samples were collected from all patients (prior to the dialysis session) and healthy controls from the antecubital vein between 6:00 AM and 8:00 AM, with the participants in a supine position after fasting for 12 hours. All participants provided informed consent, and the institution’s ethics committee approved the study (IBBU-02-2016). Investigations were performed in accordance with the ethical standards outlined in the 1975 Declaration of Helsinki, as revised in 1983.
Laboratory investigations
Serum creatinine, urea, glucose, TGs, TC, LDL-C, and HDL-C were determined on a Roche/Hitachi Cobas c311 System auto-analyzer using the CREJ2, UREAL (Urea/BUN), GLUC3, TRIGL, CHOL2, LDLC2 and HDLC3 kits, respectively (Roche Diagnostics, Manheim, Germany). Several approaches for direct measurement of HDL-C in serum have been proposed, including the use of magnetically responsive particles as polyanionmetal combinations and the use of polyethylene glycol with anti-apoprotein B and anti-apoprotein CIII antibodies. LDL-C was measured using a Cobas LDL-C assay kit intended for direct determination of LDL-C concentration in human serum and plasma using a homogeneous enzymatic colorimetric method. This automated method takes advantage of the selective micellary solubilization of LDL-C by a nonionic detergent and interaction of a sugar compound and lipoprotein (very low-density lipro-proteins and chylomicrons). The combination of a sugar compound with a detergent enables the selective determination of LDL-C in serum.
Creatine kinase MB isoenzyme assay
CK-MB was analyzed on a Cobas Integra c311 system using the Roche Method according to the recommendations of the International Federation of Clinical Chemistry (IFCC), the Société Française de Biologie Clinique (SFBC), the Committee on Enzymes of the Scandinavian Society for Clinical Chemistry and Clinical Physiology (SCE), and the German Society for Clinical Chemistry (DGKC) using the immunoinhibition method. This method requires anti-CK-M antibody, which inhibits the M subunit of CK-MB and CK-MM; eventually, the enzymatic activity of CK-B was determined with a standardized method for the determination of CK using a reverse reaction and activation by N-acetylcysteine. The activated CK catalyzes the dephosphorylation of creatine phosphate to form creatine and adenosine triphosphate (ATP). The produced ATP was used to phosphorylate glucose to form D-glucose-6-phosphate (G6P). Then, G6P was oxidized by glucose-6-phosphate dehydrogenase (G6PDH) in the presence of nicotinamide adenine dinucleotide phosphate (NADP+) to form 6-phosphogluconate and NADPH. The rate of NADPH formation is directly proportional to the activity of catalytic CK-MB and is determined by measuring the increase in absorbance at 340 nm. For healthy people, the reference range is < 25 U/L according to Klein et al [11]; therefore, a value of ≥ 25 U/L indicates abnormality.
High-sensitivity cardiac troponin T assay
Serum cardiac troponin T concentration was measured using fifth-generation hs-TnT reagents on the Elecsys 2010/cobas e411 and Modular® Analytics E170 immuno-analyzers (Roche Diagnostics) by electrochemiluminescence immunoassay (ECLIA) using a ruthenium complex (Tris(bipyridyl)-ruthenium(II)-complex) as an antibody label. According to the package insert, this highly sensitive assay has a limit of detection of 0.003 μg/L, a 99th percentile URL of 0.014 μg/L, and a 10% coefficient of variation at 0.013 μg/L. In keeping with recent studies, a value of 0.016 μg/L or higher was considered elevated; associated with cardiac structure and impaired function; and predictive of heart failure, cardiovascular death, and all-cause mortality [12,13]. The assay procedure is similar to that of the fourth-generation assay. The sample, biotinylated capture antibody (2.5 μg/mL), and ruthenium-labeled detection antibody (2.5 μg/mL) are incubated for 4.5 minutes in the homogeneous phase (Elecsys 2010/cobas e 411; STAT application). Streptavidin-coated beads are added to the the reaction mixture, followed by incubation for another 4.5-minutes to facilitate binding of the formed immune complexes to the microparticles. Then, the reaction mixture is transferred into the measuring cell, where beads are attracted to the electrode’s surface by a magnet. To remove the unbound label, the measuring cells are washed and filled with tris-propylamine contained in a detection buffer. Voltage is applied to the electrode, and the emitted chemiluminescence light is detected by a photomultiplier. Results are calculated via a two-point calibration curve that is instrument specific.
Statistical analysis
Statistical analysis was performed using independent sample test for parametric variables and chi-square test for categorical variables. Quantitative baseline characteristics are presented as mean ± standard deviation. Frequencies and proportions were generated for categorical variables. A nonparametric approach was used to calculate the URLs (99th percentiles). We performed a univariate analysis to determine the associations between cardiovascular risk factors and levels of the cardiac biomarkers CK-MB and hs-TnT. Logistic regression analysis was executed using the forward Wald selection procedure. Three binary logistic regression models were conducted to test the prognostic value of CK-MB and hs-TnT biomarkers for prediction of cardiovascular risks in hemodialysis patients. Receiver operating characteristic (ROC) analysis was used to assess the prognostic accuracy and performance of cardiac biomarkers in terms of the area under the curve (AUC). The predicted probabilities determined by the logistic regression models were used to generate an ROC curve. All tests were two-sided with statistical significance set at P < 0.05. Data were analyzed using the Statistical Package for Social Sciences, version 16.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Demographic characteristics of the study population
Out of the 120 selected participants, 110 adults who met the inclusion criteria and completed the questionnaire were included in the present study. Among the 110 study participants, 54 were ESRD patients randomly selected from adults receiving in-center hemodialysis in the Kidney Dialysis Center at Al-Thawra Hospital in Ibb, Yemen. The other 56 participants were selected as healthy controls from the Blood Bank Department at Al-Thawra Hospital. The demographic parameters of the two study groups are shown in Table 1. No significant difference was found between the hemodialysis patients and healthy controls regarding age (P = 0.886) and gender (P = 0.881). Systolic blood pressure (P < 0.001) and diastolic blood pressure (P < 0.001) values were significantly higher, whereas BMI (P < 0.001) was significantly lower in the hemodialysis patients compared with the healthy controls.
Table 1.
Demographic parameters of hemodialysis patients and healthy controls
| Characteristic | Hemodialysis patients (n = 54) | Healthy control (n = 56) | P value |
|---|---|---|---|
| Age (yr) | 43.65 ± 13.79 | 43.30 ± 11.04 | 0.886 |
| Sex, male/female | 21/33 | 21/35 | 0.881 |
| Blood pressure (mmHg) | |||
| Systolic | 144.26 ± 21.24 | 115.75 ± 14.80 | < 0.001 |
| Diastolic | 85.83 ± 11.48 | 72.68 ± 8.63 | < 0.001 |
| Body mass index (kg/m2) | 20.64 ± 3.24 | 24.79 ± 3.39 | < 0.001 |
Values are presented as mean ± standard deviation or number only.
Significantly different between the two groups if P < 0.05.
Clinical characteristics of the study populations
The clinical characteristics of the hemodialysis patients and healthy controls are summarized in Table 2. Mean glucose, urea and creatinine levels were significantly higher in the hemodialysis patients than in healthy individuals (P < 0.001). Most lipids showed significant elevation in hemodialysis patients compared to healthy controls except for HDL-C, which had a significant decrease. LDL-C and TC were elevated significantly in hemodialysis patients compared to healthy controls (95.98 ± 45.52 and 145.2 ± 52.14 mg/dL vs. 74.17 ± 35.05 and 112.92 ± 52.19 mg/dL respectively, P < 0.01). LDL-C/HDL-C and TC/HDL-C The ratios also showed a significant elevation in hemodialysis patients compared to healthy controls (5.94 ± 4.059 and 9.25 ± 6.81 vs. 2.19 ± 1.30 and 3.30 ±1.89, P < 0.001); however, a significant decrease was recorded for HDL-C (19.46 ± 10.44 vs. 37.24 ± 12.21 mg/dL, P < 0.001). The CK-MB and hs-TnT values were higher in hemodialysis patients than in healthy controls, and the difference reached statistical significance (P < 0.05). There was no difference in TG levels (P = 0.774) between the study groups.
Table 2.
Comparison of clinical parameters between hemodialysis patients and healthy controls
| Parameter | Hemodialysis patients (n = 54) | Healthy control (n = 56) | P value |
|---|---|---|---|
| Glucose (mg/dL) | 60.75 ± 18.14 | 99.36 ± 61.35 | < 0.001 |
| Urea (mg/dL) | 109.33 ± 63.31 | 18.83 ± 14.50 | < 0.001 |
| Creatinine (mg/dL) | 6.98 ± 2.84 | 0.50 ± 0.23 | < 0.001 |
| TG (mg/dL) | 91.16 ± 50.13 | 87.90 ± 61.86 | 0.774 |
| TC (mg/dL) | 145.2 ± 52.14 | 112.92 ± 52.19 | 0.002 |
| LDL-C (mg/dL) | 95.98 ± 45.52 | 74.17 ± 35.05 | 0.006 |
| HDL-C (mg/dL) | 19.46 ± 10.44 | 37.24 ± 12.21 | < 0.001 |
| LDL-C/HDL-C | 5.94 ± 4.06 | 2.19 ± 1.30 | < 0.001 |
| TC/HDL-C | 9.25 ± 6.81 | 3.30 ±1.89 | < 0.001 |
| CK-MB (U/L) | 10.21 ± 7.33 | 7.57 ± 4.87 | 0.029 |
| hs-TnT (μg/L) | 0.05 ± 0.03 | 0.00 ± 0.01 | <0.001 |
Values are presented as mean ± standard deviation.
CK-MB, creatine kinase-MB isoenzyme; HDL-C, high-density lipoprotein cholesterol; hs-TnT, high-sensitivity troponin T; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
Significantly different between the two groups if P < 0.05.
Frequency of the study population by cardiac markers levels
We categorized the study participants by the risk factors commonly used in clinical practice for CVD prediction. The frequency of higher values of parameters that lead to increased risk of CVD among the study groups is shown in Table 3. Hypertension was more common in hemodialysis patients than in healthy controls based on the cutoff points of systolic and diastolic blood pressure, with percentages of 70.4% vs. 10.7% and 55.6% vs. 3.6%, respectively, P < 0.001. The results also showed that 90.7% and 81.5% of the hemodialysis patients had high-risk LDL-C/HDL-C and TC/HDL-C ratios, respectively, and were significantly higher than these seen in healthy controls (P < 0.001). However, high values of hs-TnT were exclusively seen in hemodialysis patients, at a high percentage (96.3%). Abnormal TC, LDL-C, and CK-MB values were limited in both groups (P > 0.05).
Table 3.
Distribution of the study groups by the cutoff point for high cardiovascular risk
| Parameter | Hemodialysis patients (n=54) | Healthy control (n=56) | Chi-square | P value |
|---|---|---|---|---|
| Hypertension | 52 (96.3) | 5 (8.9) | 84.05 | < 0.0001 |
| Dyslipidemia | 50 (92.6) | 31 (55.4) | 22.14 | < 0.001 |
| SBP, ≥ 140 mmHg | 38 (70.4) | 6 (10.7) | 40.77 | < 0.001 |
| DBP, ≥ 90 mmHg | 30 (55.6) | 2 (3.6) | 36.01 | < 0.001 |
| TG, ≥ 200 mg/dL | 6 (11.1) | 6 (10.7) | 0.004 | 0.947 |
| TC, ≥ 240 mg/dL | 1 (1.9) | 2 (3.6) | 0.306 | 0.580 |
| HDL-C, < 40 mg/dL | 50 (92.6) | 31 (55.4) | 19.63 | < 0.001 |
| LDL-C, ≥ 160 mg/dL | 1 (1.9) | 1 (1.8) | 0.001 | 0.979 |
| LDL-C/HDL-C, ≥ 4 | 49 (90.7) | 16 (28.6) | 43.95 | < 0.001 |
| TC/HDL-C, ≥ 6 | 44 (81.5) | 13 (23.2) | 37.38 | < 0.001 |
| CK-MB, ≥ 25 U/L | 3 (5.6) | 2 (3.6) | 0.249 | 0.617 |
| hs-TnT, ≥ 0.016 μg/L | 52 (96.3) | 0 (0) | 102.3 | < 0.001 |
Values are presented as number (%).
CK-MB, creatine kinase-MB isoenzyme; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; hs-TnT, high-sensitivity troponin T; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
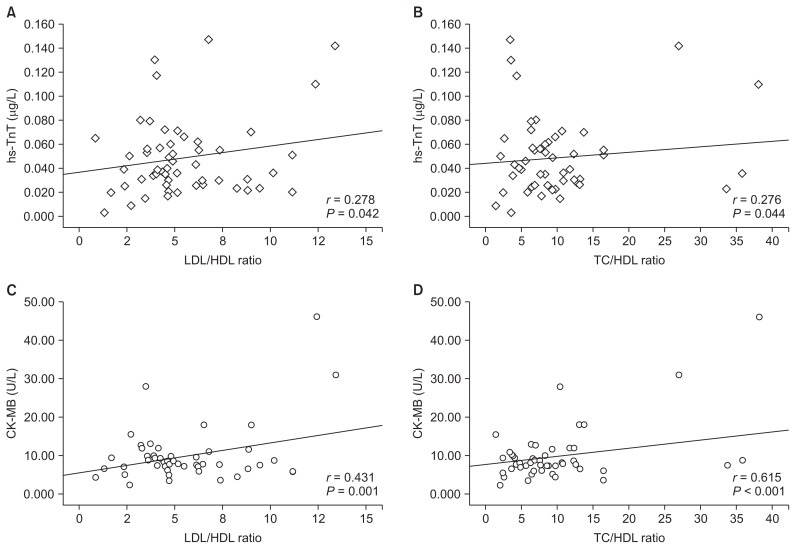
Correlations of hs-TnT and CK-MB with other parameters in ESRD patients
Univariate associations using CK-MB and hs-TnT as the dependent variables are shown in Table 4 and Fig. 1. Serum CK-MB was significantly and positively correlated with LDL-C (r = 0.420, P = 0.002), LDL-C/HDL-C (r = 0.431, P = 0.001), and TC/HDL-C (r = 0.615, P < 0.001). Serum hs-TnT showed results generally comparable with CK-MB, with a significant correlation with LDL-C (r = 0.317, P = 0.02), LDL-C/HDL-C (r = 0.278, P = 0.042), and TC/HDL-C (r = 0.27, P = 0.044). CK-MB plasma level was negatively correlated with HDL-C (r = −0.344, P = 0.011), while hs-TnT level did not show significant association with HDL-C in Pearson’s correlation (P = 0.46). Neither CK-MB nor hs-TnT value was correlated with BMI and TC levels.
Table 4.
Associations between CK-MB and hs-TnT as continuous variables and cardiovascular risk factors in ESRD patients on hemodialysis
| Variable | CK-MB | hs-TnT | ||
|---|---|---|---|---|
|
|
|
|||
| r | P | r | P | |
| BMI | 0.056 | 0.68 | 0.05 | 0.719 |
| HDL-C | −0.344 | 0.011* | −0.103 | 0.46 |
| LDL-C | 0.420 | 0.002* | 0.317 | 0.02* |
| TC | 0.174 | 0.208 | 0.069 | 0.622 |
| LDL-C/HDL-C | 0.431 | 0.001* | 0.278 | 0.042* |
| TC/HDL-C | 0.615 | < 0.001* | 0.276 | 0.044* |
BMI, body mass index; CK-MB, creatine kinase-MB isoenzyme; DBP, diastolic blood pressure; ESRD, end-stage renal disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol.
r, Pearson’s correlation coefficient;
the difference is significant if P < 0.05.
Figure 1.
Scatterplot depicting the relationships between hs-TnT and CK-MB values with the LDL-C/HDL-C and TC/HDL-C ratios in all hemodialysis patients.
hs-TnT, high-sensitivity cardiac troponin T; CK-MB, creatine kinase MB isoenzyme; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol.
Associations of CK-MB and hs-TnT with CV risk factors in ESRD patients
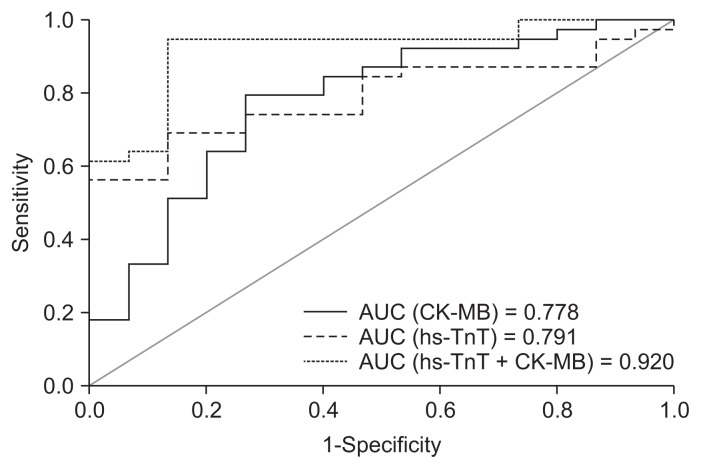
A logistic regression analysis was conducted based on the presence or absence of hypertension as a major risk factor for CVDs. The risk factors, which had a significant association with hs-TnT and CK-MB in the univariate analysis (HDL-C, LDL-C, LDL-C/HDL-C, TC/HDL-C), as well as CK-MB and hs-TnT, were used as the independent variables. Three models were included in the logistic regression analysis as shown in Table 5. Overall, 74.1% of the patients were predicted correctly by the first model (Model 1) containing CVD risk factors and hs-TnT, and 68.5% of the patients were correctly classified by the second model (Model 2) in which CK-MB replaced hs-TnT. However, 90.7% of the patients were predicted correctly by Model 3 containing CVD risk factors and both hs-TnT and CK-MB. After examining the results of Model 3, only hs-TnT (odds ratio [OR], 2.153; 95% confidence interval [CI], 1.336–2.961; P = 0.017) and CK-MB (OR, 1.138; 95% CI, 1.006–1.288; P = 0.04) were found to have significant associations with CVD (Table 5). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of hs-TnT and CK-MB are shown in Table 6. The addition of CK-MB to hs-TnT did improve the specificity, PPV, or NPV of the prognostic test for predicting cardiovascular events in our patients, with values of 86.7%, 94.7%, and 81.3%, respectively. ROC curves for hs-TnT and CK-MB alone and the combination of the two biomarkers provided the AUC, as depicted in Fig. 2. The combination of hs-TnT and CK-MB resulted in the highest AUC (0.920) with a more convex shape compared to hs-TnT or CK-MB alone.
Table 5.
Multivariate statistical models of the factors that predict cardiovascular risk in hemodialysis patients using logistic regression analysis
| β | SE | Wald | OR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Constant | 0.652 | 1.349 | ||||
| HDL-C | −0.031 | 0.051 | 0.369 | 0.970 | 0.878–1.071 | 0.544 |
| LDL-C | 0.029 | 0.018 | 2.490 | 1.029 | 0.993–1.067 | 0.115 |
| LDL-C/HDL-C | 0.521 | 0.347 | 2.253 | 0.594 | 0.300–1.173 | 0.133 |
| TC/HDL-C | 0.103 | 0.078 | 1.740 | 1.108 | 0.951–1.291 | 0.187 |
| hs-TnT | 2.182 | 1.907 | 5.134 | 5.822 | 1.525–2.222 | 0.023* |
| Model 2 | ||||||
| Constant | −0.551 | 1.380 | ||||
| HDL-C | −0.012 | 0.052 | 0.057 | 0.893 | 0.988–1.093 | 0.812 |
| LDL-C | 0.004 | 0.016 | 0.074 | 1.004 | 0.973–1.036 | 0.785 |
| LDL-C/HDL-C | 0.036 | 0.283 | 0.016 | 0.554 | 0.965–1.680 | 0.900 |
| TC/HDL-C | 0.017 | 0.064 | 0.073 | 0.866 | 0.983–1.115 | 0.787 |
| CK-MB | 0.118 | 0.058 | 4.182 | 1.005 | 1.125–1.259 | 0.041* |
| Model 3 | ||||||
| Constant | −2.408 | 1.652 | ||||
| HDL-C | −0.040 | 0.063 | 0.404 | 0.960 | 0.848–1.088 | 0.525 |
| LDL-C | 0.024 | 0.023 | 1.069 | 1.024 | 0.979–1.072 | 0.301 |
| LDL-C/HDL-C | 0.513 | 0.421 | 1.483 | 0.599 | 0.262–1.367 | 0.223 |
| TC/HDL-C | 0.087 | 0.094 | 0.859 | 1.091 | 0.907–1.313 | 0.354 |
| hs-TnT | 1.974 | 1.073 | 5.661 | 2.153 | 1.336–2.961 | 0.017* |
| CK-MB | 0.129 | 0.063 | 4.216 | 1.138 | 1.006–1.288 | 0.040* |
β, regression coefficient; CI, confidence interval; CK-MB, creatine kinase-MB iso-enzyme; HDL-C, high-density lipoprotein cholesterol; hs-TnT, high-sensitivity troponin T; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; SE, standard error; TC, total cholesterol.
Significant difference.
Table 6.
Test performance of cardiovascular event prediction with CK-MB, hs-TnT, and hs-TnT + CK-MB
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| CK-MB | 87.2 | 20.0 | 73.9 | 37.5 |
| hs-TnT | 92.3 | 26.7 | 76. 6 | 57.1 |
| hs-TnT + CK-MB | 92.3 | 86.7 | 94.7 | 81.3 |
CK-MB, creatine kinase-MB isoenzyme; hs-TnT, high-sensitivity troponin T; NPV, negative predictive value; PPV, positive predictive value.
Figure 2.
The receiver-operating characteristic curve shows the area under the curve (AUC) values for CK-MB, hs-TnT, and the combination of hs-TnT and CK-MB.
hs-TnT, high-sensitivity cardiac troponin T; CK-MB, creatine kinase MB isoenzyme.
Discussion
The 110 individuals included in our study represent 90.0% of all selected outpatients on hemodialysis and 93.3% of healthy participants during the data collection period in the Ibb province of Yemen. With the exception of TGs, the demographic and clinical data of the present study were significantly different between ESRD patients and healthy controls. BMI results were significantly decreased in hemodialysis patients compared to healthy controls, suggesting that patients with low BMI are at high risk of morbidity. These findings are in line with previous studies, which demonstrated that increased BMI is associated with better survival, whereas low BMI and weight loss are associated with increased mortality (the so-called obesity paradox) [14,15]. Both cardiovascular biomarkers (hs-TnT and CK-MB) were significantly higher in hemodialysis patients than in healthy controls. The participants were categorized into two cardiovascular risk groups based on the above thresholds of the cardiovascular risk factors and referred to as high risk. Using this simple clinical stratification, we found large differences in CV risk between the two groups, as revealed in Table 3.
Hypertension was found in 96.3% of participants, which is similar to the results found in the CHOICE study [16] but higher than the 87.1% in the study by Ohsawa et al [17] in Japan and the 86% reported in a multicenter cohort in Spain [18]. The deposition of lipids in the kidney can cause kidney damage in a manner analogous to lipid deposition in the vascular wall in atherosclerosis development [19]. The incidence of dyslipidemia was 92.6% in our patients, which was higher than that reported by other studies, such as 50% in the study of Longenecker et al [16]. Our mean HDL-C was 19.46 mg/dL, and HDL-C under 40 mg/dL was more frequently observed in our study (92.2%) than in others [16–18]. The cardiovascular risk indices TC/HDL-C and LDL-C/HDL-C of the ESRD patients were significantly higher than those of the healthy control group, which is in line with the study of Chijioke et al [20]. The prevalence of increased TC/HDL-C (81.5%) and LDL-C/HDL-C (90.7%) ratios in ESRD patients on hemodialysis confirmed that these ratios were more specific and accurate indices for assessing cardiovascular risk than considering only TC, TG, HDL-C, or LDL-C level alone.
The present study aimed to explore the prognostic usefulness of hs-TnT alone or in combination with the CK-MB isoenzyme in the prediction of cardiovascular risks in asymptomatic ESRD patients on hemodialysis. Although CK-MB concentrations are higher in ESRD patients undergoing hemodialysis than in healthy people, the frequency of elevated levels is limited and not different in the two groups. This conclusion is similar to previous findings reported by Fredericks et al [21], who showed that CK-MB is unaffected by uremia in humans. In a study by Lal et al [22], 22 hemodialysis patients demonstrated increased total CK values, which corresponded to 42% of the group, while 6 of these 22 hemodialysis patients (27%) had elevated CK-MB isoenzyme greater than 5%. In contrast, hs-TnT was significantly higher in hemo-dialysis patients than in healthy participants with a high prevalence of positive values (96.3%) in ESRD patients on maintenance hemodialysis. The data from our study are consistent with other studies, which reported elevated troponin levels in patients without acute coronary syndromes and with normal CK-MB level [23,24].
There is emerging evidence that increased troponin T concentration in asymptomatic patients with ESRD indicates subclinical myocardial necrosis or injury. In their study on long-term hemodialysis patients, deFilippi et al [25] found that degree of troponin T elevation was significantly correlated with extent and severity of angiographic coronary artery disease. Our study is supported by other studies showing the usefulness of hs-TnT in hemodialysis populations. These studies have confirmed usual elevations of hs-TnT to be related to renal impairment [26,27]. Cardiac troponin T has been also shown to correlate with degree of coronary artery calcification in asymptomatic HD patients [28]. Overall, these findings suggest that monitoring troponin T concentrations can help to refine cardiovascular risk stratification in hemodialysis patients.
Univariate correlation analysis shows that both hs-TnT and CK-MB concentrations correlate positively and significantly with LDL-C, LDL-C/HDL-C, and TC/HDL-C, with the strongest correlation seen between CK-MB and TC/HDL-C (r = 0.615, P < 0.001). All cardiovascular risk factors that show significant correlation with either hs-TnT or CK-MB, listed in Table 3, are entered into stepwise logistic regression models. The strongest model with the highest Wald chi-square statistic (Wald = 5.661, P = 0.017) contains both biomarkers, CK-MB and hs-TnT. The ROC curve, which is defined as a plot of test sensitivity versus its 1-specificity, was used to describe and compare the performance of these biomarkers.
The two-biomarker model has an AUC of 0.920 (P < 0.0001), which is greater than those of the single-biochemical marker models. Furthermore, hs-TnT shows high prognostic sensitivity (92.3%) and PPV (76.6%) compared to those for CK-MB; 87.2% and 73.9%, respectively (Table 6). Although the enzyme CK-MB is less specific than hs-TnT, the specificity of combined hs-TnT and CK-MB as biomarkers of myocardial injury among patients with ESRD has been greatly enhanced. The single markers have an AUC of 0.791 and 0.778 for hs-TnT and CK-MB, respectively. The analysis demonstrates that 1) hs-TnT has superior discriminative ability for CVD against CK-MB, 2) the predefined cut-off value of 0.016 μg/L improves the diagnostic capacity and effectiveness of hs-TnT to identify diseased subjects with excellent prognosis, and 3) the two biomarkers have comparable prognostic accuracy and can provide a better discriminating tool for CVD events. Troponin T seems to be a powerful prognostic biomarker for detecting cardiac injury in ESRD patients on hemodialysis [29–31]. Though troponins are being increasingly used because of their advantages over CK-MB, selective situations warrant CK-MB as the preferred marker. For example, Quiroga et al [32] have recently demonstrated that CK-MB is a good marker for stratifying cardiovascular risk in ESRD patients receiving long-term hemodialysis.
In conclusion, hs-TnT appears to be an important predictor for cardiovascular mortalities in this population. Although studies suggest that CK-MB has relatively low sensitivity and specificity for identifying cardiovascular events in hemodialysis patients, others found that CK-MB is a good marker for risk stratification in hemodialysis patients even in the normal range. No studies have elucidated the role of CK-MB in the augmentation of the prognostic potential and accuracy of hs-TnT in asymptomatic dialysis recipients. Our data show the better prognostic accuracy and improved predictive value of the combination of hs-TnT and CK-MB compared with hs-TnT alone. These observations will provide new insights into the predictive value of multiple biomarkers for identifying ESRD patients on chronic hemodialysis at highest risk for CV events.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.United States Renal Data System (USRDS) USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2009. [Google Scholar]
- 2.Chijioke A, Makusidi AM, Shittu AO, et al. Pattern of lipid profile in dialysis naive chronic kidney disease patients from Ilorin, Nigeria. Internet J Nephrol. 2010;6:1–6. [Google Scholar]
- 3.Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci. 2003;325:163–167. doi: 10.1097/00000441-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Paraskevas KI, Kotsikoris I, Koupidis SA, Tzovaras AA, Mikhailidis DP. Cardiovascular events in chronic dialysis patients: emphasizing the importance of vascular disease prevention. Int Urol Nephrol. 2010;42:999–1006. doi: 10.1007/s11255-010-9795-7. [DOI] [PubMed] [Google Scholar]
- 5.Collins AJ, Foley RN, Chavers B, et al. US Renal data system 2013 annual data report. Am J Kidney Dis. 2014;63(1 Suppl):A7. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Roberts MA, Hare DL, Ratnaike S, Ierino FL. Cardiovascular biomarkers in CKD: pathophysiology and implications for clinical management of cardiac disease. Am J Kidney Dis. 2006;48:341–360. doi: 10.1053/j.ajkd.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Kirkendall WM, Burton AC, Epstein FH, Freis ED. Recommendations for human blood pressure determination by sphygmomanometers. Circulation. 1967;36:980–988. doi: 10.1161/01.CIR.36.6.980. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 9.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 11.Klein G, Berger A, Bertholf R, et al. Multicenter evaluation of liquid reagents for CK, CK-MB and LDH with determination of reference intervals on HitachiSsystems. Clin Chem. 2001;47(Suppl):A30. [Google Scholar]
- 12.Saenger AK, Beyrau R, Braun S, et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta. 2011;412:748–754. doi: 10.1016/j.cca.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Streja E, Kovesdy CP, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JC, Koo KT, Lim HC. The hidden X suture: a technical note on a novel suture technique for alveolar ridge preservation. J Periodontal Implant Sci. 2016;46:415–425. doi: 10.5051/jpis.2016.46.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002;13:1918–1927. doi: 10.1097/01.ASN.0000019641.41496.1E. [DOI] [PubMed] [Google Scholar]
- 17.Ohsawa M, Kato K, Itai K, et al. Cardiovascular risk factors in hemodialysis patients: results from baseline data of kaleidoscopic approaches to patients with end-stage renal disease study. J Epidemiol. 2005;15:96–105. doi: 10.2188/jea.15.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-García R, Martín-Malo A, Fort J, et al. Baseline characteristics of an incident haemodialysis population in Spain: results from ANSWER--a multicentre, prospective, observational cohort study. Nephrol Dial Transplant. 2009;24:578–588. doi: 10.1093/ndt/gfn464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal R. Effects of statins on renal function. Mayo Clin Proc. 2007;82:1381–1390. doi: 10.4065/82.11.1381. [DOI] [PubMed] [Google Scholar]
- 20.Chijioke NN, Bartimaeus EAS, Okeke CU. Lipid profile in chronic renal failure patients on dialysis. Eur J Cardiovasc Med. 2012;2:106–109. [Google Scholar]
- 21.Fredericks S, Murray JF, Bewick M, et al. Cardiac troponin T and creatine kinase MB are not increased in exterior oblique muscle of patients with renal failure. Clin Chem. 2001;47:1023–1030. [PubMed] [Google Scholar]
- 22.Lal SM, Nolph KD, Hain H, et al. Total creatine kinase and isoenzyme fractions in chronic dialysis patients. Int J Artif Organs. 1987;10:72–76. [PubMed] [Google Scholar]
- 23.van Bockel EA, Tulleken JE, Ligtenberg JJ, van der Werf TS, Aarts LP, Zijlstra JG. [The significance of elevated troponin levels in the absence of acute cardiac ischaemia]. Ned Tijdschr Geneeskd. 2005;149:1879–1883. In Dutch. [PubMed] [Google Scholar]
- 24.Hamm CW, Giannitsis E, Katus HA. Cardiac troponin elevations in patients without acute coronary syndrome. Circulation. 2002;106:2871–2872. doi: 10.1161/01.CIR.0000044342.50593.63. [DOI] [PubMed] [Google Scholar]
- 25.deFilippi C, Wasserman S, Rosanio S, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290:353–359. doi: 10.1001/jama.290.3.353. [DOI] [PubMed] [Google Scholar]
- 26.McGill D, Talaulikar G, Potter JM, Koerbin G, Hickman PE. Over time, high-sensitivity TnT replaces NT-proBNP as the most powerful predictor of death in patients with dialysis-dependent chronic renal failure. Clin Chim Acta. 2010;411:936–939. doi: 10.1016/j.cca.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Ye P, Luo L, Xu R, Bai Y, Wu H. Association of glomerular filtration rate with high-sensitivity cardiac troponin T in a community-based population study in Beijing. PLoS One. 2012;7:e38218. doi: 10.1371/journal.pone.0038218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung HH, Ma KR, Han H. Elevated concentrations of cardiac troponins are associated with severe coronary artery calcification in asymptomatic haemodialysis patients. Nephrol Dial Transplant. 2004;19:3117–3123. doi: 10.1093/ndt/gfh488. [DOI] [PubMed] [Google Scholar]
- 29.Deegan PB, Lafferty ME, Blumsohn A, Henderson IS, Mc-Gregor E. Prognostic value of troponin T in hemodialysis patients is independent of comorbidity. Kidney Int. 2001;60:2399–2405. doi: 10.1046/j.1523-1755.2001.00076.x. [DOI] [PubMed] [Google Scholar]
- 30.Conway B, McLaughlin M, Sharpe P, Harty J. Use of cardiac troponin T in diagnosis and prognosis of cardiac events in patients on chronic haemodialysis. Nephrol Dial Transplant. 2005;20:2759–2764. doi: 10.1093/ndt/gfi125. [DOI] [PubMed] [Google Scholar]
- 31.Satyan S, Light RP, Agarwal R. Relationships of N-terminal pro-B-natriuretic peptide and cardiac troponin T to left ventricular mass and function and mortality in asymptomatic hemodialysis patients. Am J Kidney Dis. 2007;50:1009–1019. doi: 10.1053/j.ajkd.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quiroga B, Vega A, Abad S, Villaverde M, Reque J, López-Gómez JM. Creatine-kinase and dialysis patients, a helpful tool for stratifying cardiovascular risk? Nefrologia. 2016;36:51–56. doi: 10.1016/j.nefro.2015.10.004. [DOI] [PubMed] [Google Scholar]