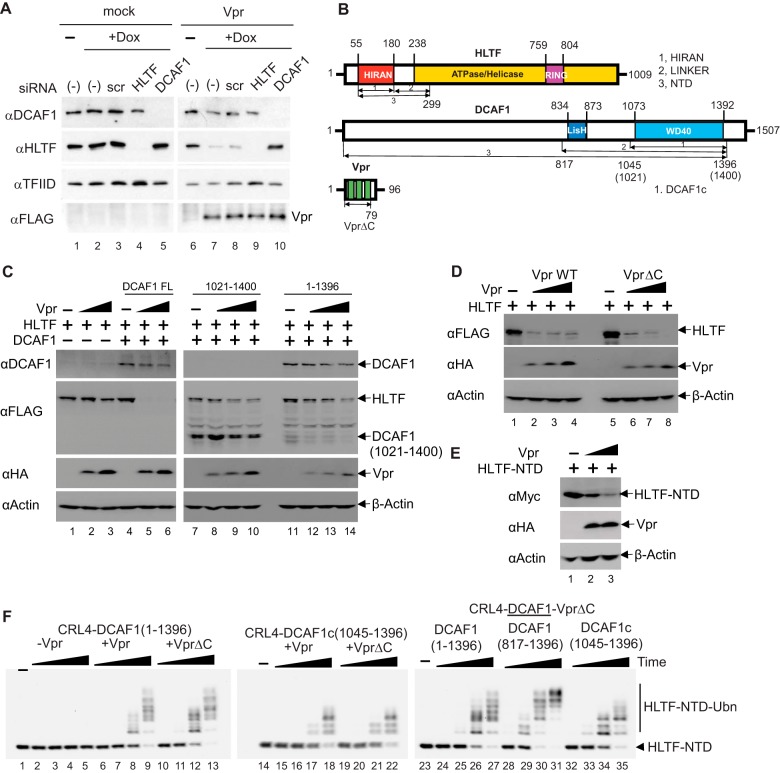
Figure 1.
HIV-1 Vpr directly loads HLTF onto CRL4-DCAF1 to mediate its proteasome-dependent degradation. A, U2OS cells harboring doxycycline (Dox) inducible HIV-1 Vpr transgene were transfected with scrambled (scr) siRNA or siRNA targeting HLTF or DCAF1 and treated with doxycycline for 48 h. Cell extracts were analyzed by immunoblotting with antibodies reacting with DCAF1, HLTF, and FLAG-tagged Vpr. TFIID was used as a loading control. B, domain organization of HLTF, DCAF1, and Vpr. HLTF comprises a single-stranded DNA–binding HIRAN domain, an ATPase domain, and a RING domain. Recombinant HLTF-HIRAN (residues 55–180), LINKER (residues 181–299), and NTD (residues 55–299) constructs were prepared for in vitro reconstitution assays. DCAF1 contains a LisH dimerization domain and a WD40 domain followed by a stretch of acidic amino acid residues. Recombinant DCAF1 constructs (residues 1045–1396, henceforth DCAF1c; 817–1396; 1–1396) were prepared for in vitro assays. Alternatively, a DCAF1 construct of residues 1021–1400 was transiently transfected into HEK 293T cells. Vpr comprises three α helices within residues 16–32, 36–51, and 53–74 (39). Recombinant WT and VprΔC (residues 1–79) constructs were prepared. C, constant amounts of plasmids expressing HLTF and the indicated DCAF1 constructs (full-length (FL), residues 1021–1400, and residues1–1396) were transiently co-transfected into HEK 293T cells together with increasing amounts of HIV-1 Vpr expression plasmid. At 48 h post transfection, cells were harvested and analyzed by Western blotting. D, HEK 293T cells were transfected with a constant amount of plasmids expressing full-length HLTF and DCAF1, and increasing amounts of a plasmid expressing Vpr WT or VprΔC. E, constant amounts of HLTF-NTD (residues 55–299) expression plasmid were transiently co-transfected with increasing amounts of Vpr expression plasmid into HEK 293T cells. F, in vitro ubiquitination assays of HLTF-NTD with CRL4-DCAF1, CRL4-DCAF1-Vpr, or CRL4-DCAF1-VprΔC, where DCAF1 constructs vary (residues 1–1396, 816–1396, or 1045–1396) as indicated. Polyubiquitinated HLTF species are indicated (HLTF-NTD-Ubn). All experiments were repeated three times with equivalent results.