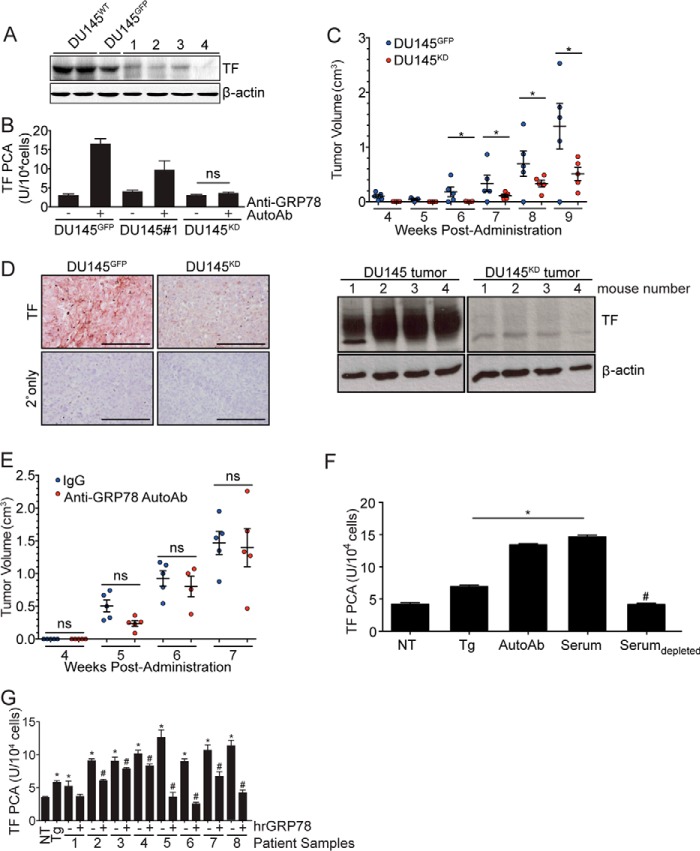
Figure 4.
TF downmodulation in DU145 cells inhibits anti-GRP78 AutoAb-mediated TF activity and tumor progression. A, Western blot analysis of TF expression in cells transduced with lentiviral particles encoding TF shRNA. Control cells were transduced with lentiviral particles encoding GFP (DU145GFP). β-Actin was used as a loading control. Clone 4 demonstrates high efficiency of TF knockdown (DU145KD). B, TF procoagulant (PCA) activity is induced by the anti-GRP78 AutoAbs and is proportional to TF expression levels (*, p < 0.05 AutoAbs (+) versus non-treated (−) cells; n = 5). C, in vivo, DU145KD xenografts grow more slowly than DU145GFP xenografts (*, p < 0.05). D, IHC analysis demonstrates lower TF expression in DU145KD xenografts, compared with DU145GFP (left); TF total protein amounts were lower in the DU145 TFKD animal group versus control (Western blotting; right). Scale bar, 100 μm. Mean tumor volume ± S.D. (error bars) is shown. E, anti-GRP78 AutoAb treatment (60 μg/ml) does not alter the rate of tumor growth in DU145KD xenografts. Human IgG (60 μg/ml) treatment was used as a control (n = 5/group). Mean tumor volumes ± S.D. are shown. ns, not significant. F, treatment of DU145 cells with Tg (5 μm), anti-GRP78 AutoAbs (60 μg/ml), or patient sera increases TF activity, whereas immunodepletion of anti-GRP78 AutoAbs prevents TF activity (*, p < 0.05 versus NT cells; #, p < 0.05 versus AutoAb treatment; n = 5/group). G, pretreatment of sera from prostate cancer patients with human recombinant GRP78 (hrGRP78) (60 μg/ml) prevents the activation of TF in DU145 cells (*, p < 0.05 versus NT cells; #, p < 0.05 versus corresponding native serum; n = 5/group).