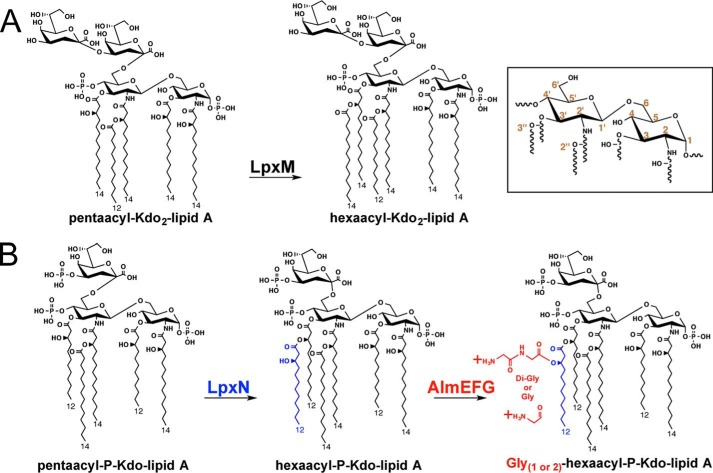
Figure 1.
Multiple differences in the predominant chemical structures of Kdo-lipid A domains of E. coli K-12 compared with V. cholerae biotype El Tor. Inset provides numeric classification legend for describing acyl chain position along the glucosamine disaccharide. A, E. coli possess a bi-functional Kdo transferase that transfers individual Kdo sugars to lipid IVA and Kdo-lipid IVA successively. The lipid A secondary acyltransferase LpxL and then LpxM acyltransferases produce the predominantly observed hexaacyl-Kdo2-lipid A. B, V. cholerae Kdo-lipid A domains contain hydroxylaurate chains at 3- and 3′-positions. V. cholerae expresses a monofunctional KdtA that transfers a single Kdo residue to lipid IVA and a Kdo kinase that phosphorylates Kdo-lipid IVA. V. cholerae LpxL (Vc0213) transfers a myristate (C14:0) to the 2′-position hydroxylacyl chain of the glucosamine disaccharide. LpxN (Vc0212) transfers a 3-hydroxylaurate (3-OH C12:0; blue) to the 3′-position hydroxyacyl chain to generate hexaacyl-monophosphoryl-Kdo lipid A. AlmEFG adds glycine to the 3-hydroxylaurate of hexaacyl-monophosphoryl-Kdo lipid A. Glycine and diglycine modified hexaacyl-monophosphoryl-Kdo-lipid A are highly abundant in V. cholerae biotype El Tor under standard growth conditions.