Figure 8.
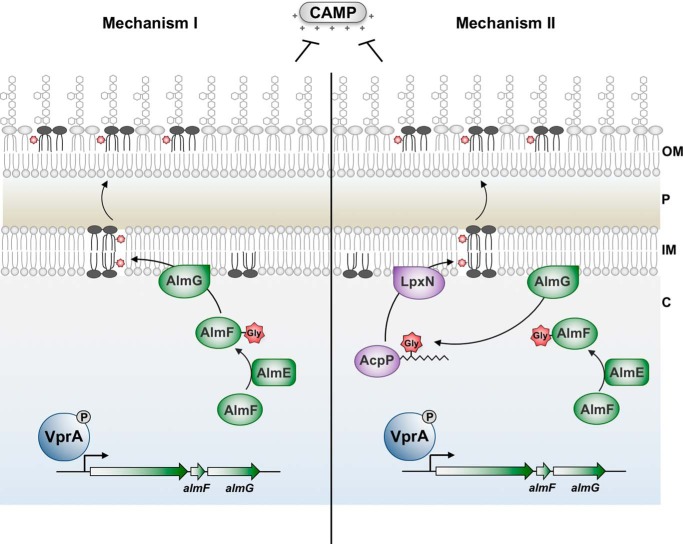
Unusual AlmG catalytic residues suggest an alternative mechanism for synthesis of glycine-modified Kdo-lipid A domains. Both hypothetical mechanisms begin with phosphorylated VprA directly promoting expression of the almEFG operon. In a two-step catalytic mechanism, AlmE (Vc1579) generates glycyl-AMP from glycine and ATP to activate glycine for transfer onto carrier protein holo-AlmF (Vc1578). Holo-AlmF is generated after 4′-phosphopantetheinyl of coenzyme A is transferred onto apo-AlmF by the phosphopantetheinyl transferase AcpS (Vc2457). In mechanism I (left), AlmG at the inner membrane uses glycyl-AlmF as the aminoacyl donor for transfer onto the secondary hydroxylauryl acyl chain of V. cholerae hexaacylated-monophosphoryl-Kdo-lipid A. At least two rounds of glycine transfer can occur. In mechanism II (right), AlmG uses glycyl-AlmF to transfer glycine onto hydroxyl-lauryl acyl carrier protein (AcpP; Vc2020). Two rounds of glycine transfer can occur. LpxN then transfers glycyl-3-hydroxylaurate from AcpP onto pentaacylated-monophosphoryl-Kdo-lipid A. In either mechanism, diglycine or glycine-modified lipid A is then transported to the bacterial surface to provide resistance against cationic antimicrobial peptides such as polymyxin. OM, outer membrane; P, periplasm; IM, inner membrane.