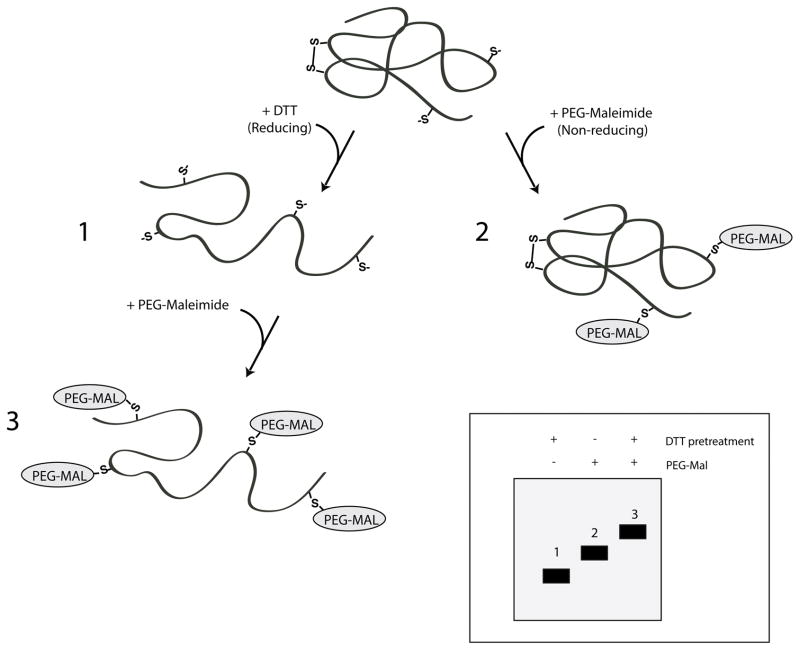
Figure 14.X.X.
Assessing oxidative state using PEG-Maleimide modification. Cartoon representation of a protein possessing two cysteines involved in an intramolecular disulfide bond and two unpaired cysteines. The presence of free thiols is analyzed using sulfhydryl-reactive maleimide linked to a polyethylene glycol group (PEG-Mal). Cells expressing the protein of interest are treated with or without DTT to assess reducing and non-reducing states, respectively (1 and 2). Cells are lysed in the presence and absence of PEG-maleimide and protein samples are resolved by SDS-PAGE. Insert, reduced and unlabeled proteins are expected to migrate fastest through the SDS-PA gel (1), followed by nonreduced proteins labeled with PEG-maleimide (2), with reduced and fully labeled proteins being slowest (3).