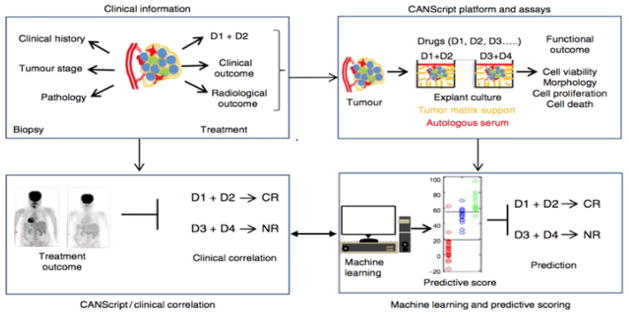
Figure 1.
CANscript® platform technology. Four critical modules were integrated in generating and validating the CANscript platform. The first module involved collecting tumor core or surgical biopsy with tumor staging and/or pathology information besides clinical history. In the second module, tumor biopsy was rapidly processed into thin explants. The explants were cultured with tumor- and grade-matched TMPs and autologous serum (AS) and incubated with selected drug regimens. While multiple drug regimens can be used, the one used by the oncologist for the patient was always included in the tumor explant culture. The in vitro functional outcome of treatment in terms of cell viability, pathological and morphological analysis, cell proliferation, and cell death was quantified. In module three, these quantitative scores from the explants were aggregated using a machine learning algorithm to assign a final score, which helped rank the outcomes as complete response (CR), partial response (PR), or no response (NR). In the final module, these predictions were tested against clinical outcomes. D1, D2, D3, and D4 indicate different drug regimens (image courtesy of Mitra RxDx).