Abstract
The quantity and quality of bone depends on osteoblastic differentiation of mesenchymal stem cells (MSCs), where adipogenic commitment depletes the available pool for osteogenesis. Cell architecture influences lineage decisions, where interfering with cytoskeletal structure promotes adipogenesis. Mechanical strain suppresses MSC adipogenesis partially through RhoA driven enhancement of cytoskeletal structure. To understand the basis of force-driven RhoA activation, we considered critical GEFs (activators) and GAPs (inactivators) on bone marrow MSC lineage fate. Knockdown of LARG accelerated adipogenesis and repressed basal RhoA activity. Importantly, mechanical activation of RhoA was almost entirely inhibited following LARG depletion, and the ability of strain to inhibit adipogenesis was impaired. Knockdown of ARHGAP18 increased basal RhoA activity and actin stress fiber formation, but did not enhance mechanical strain activation of RhoA. ARHGAP18 null MSCs exhibited suppressed adipogenesis assessed by Oil-Red-O staining and Western blot of adipogenic markers. Furthermore, ARHGAP18 knockdown enhanced osteogenic commitment, confirmed by alkaline phosphatase staining and qPCR of Sp7, Alpl, and Bglap genes. This suggests that ARHGAP18 conveys tonic inhibition of MSC cytoskeletal assembly, returning RhoA to an “off state” and affecting cell lineage in the static state. In contrast, LARG is recruited during dynamic mechanical strain, and is necessary for mechanical suppression of adipogenesis. In summary, mechanical activation of RhoA in mesenchymal progenitors is dependent on LARG, while ARHGAP18 limits RhoA delineated cytoskeletal structure in static cultures. Thus, on and off GTP exchangers work through RhoA to influence MSC fate and responses to static and dynamic physical factors in the microenvironment.
Keywords: LARG, ARHGAP18, RhoA, Mesenchymal stem cells, Adipogenesis, Osteogenesis
Introduction
Bone marrow mesenchymal stem cells (MSCs) contribute to bone structure and strength by serving as a pool for osteoprogenitors. Conditions of disuse, such as bed rest, immobilization due to disability, or a sedentary lifestyle increase marrow adiposity[1]. Conversely, exercise in vivo, as well as mechanical loading in vitro, are associated with a reduction in marrow adipocytes and increased bone formation [2–9]. These changes are attributed in part to mechanical biasing of MSCs away from the adipogenic lineage, providing a larger number of cells available for osteogenesis[10]. While there are numerous cues, including hormonal and spatial signaling that govern MSC fate, mechanical force activates several critical pathways leading to adipogenic repression[11]. A central regulator of MSC fate is β-catenin, which promotes maintenance of pluripotency[12]. We have shown that mechanical strain prevents proteasomal degradation of β-catenin by phosphorylation of GSK3-β, thus allowing β-catenin to restrict adipogenic gene transcription[13]. Furthermore, activation of GSK3-β requires phosphorylation by Akt[14], a direct substrate of the Rictor-associated mTor complex mTORC2[15]. In addition to preservation of β-catenin, our lab showed that activation of Akt/mTORC2 was necessary for downstream RhoA activity, a critical regulator of actin stress fiber formation, originating at focal adhesions (FAs)[5].
Mechanical activation of mTORC2 requires tension across FAs. Disruption of FAs impairs mTORC2/Akt signaling and leads to increased adipogenic differentiation[16]. Conversely, mechanical strain increases numbers of FAs, thus amplifying downstream signaling[17]. We have shown that signal amplification is preceded by spatial recruitment of both Fyn and FAK to FAs, where coordinated activation of mTORC2 occurs at the cell membrane[5]. Thus, mechanical force acts through FAs by compartmentalizing signaling effectors to sites of force transmission to activate β-catenin, leading to decreased adipogenic commitment. In addition to converging on β-catenin, mTORC2 signaling is necessary for mechanical assembly of actin stress fibers, in both MSCs [5, 16] and other cell types [18, 19].
The actin cytoskeleton creates a framework, spanning FA contact points, through which diverse functions take place. Actin stress fibers serve as a structural scaffold, through which cellular stiffness is regulated and intracellular signals move, dock, and activate downstream signaling[20]. Static mechanical cues, such as alteration of substrate stiffness or changing cell shape, influence MSC fate, and are partially dependent on RhoA-directed development of cytoskeletal structure [21]. In a similar fashion, dynamic application of mechanical force also activates RhoA to enhance actin stress fiber formation[5, 7]. We have shown that mTORC2/Akt signaling is required for mechanical activation of RhoA in MSCs[5]; however, the direct regulators of RhoA activation in response to dynamic mechanical strain remain unknown.
RhoA is a GTPase, inactive when guanine diphosphate (GDP) is bound and active when it binds guanine triphosphate (GTP) [22]. The on/off state of RhoA is governed by guanine nucleotide exchange factors (GEFs), which promote the GTP-bound active state, and GTPase activating proteins (GAPs), which stimulate GTP hydrolysis, resulting in GDP-bound (inactivate ) RhoA[23]. Previous work has shown that stimulation of integrins with tensional force in mouse embryonic fibroblasts (MEFs) induced recruitment of two GEFs to FA complexes, leukemia associated RhoGEF (LARG) and GEF-H1 with subsequent RhoA activation [24]. Activation of LARG required the Src-like kinase Fyn, while GEF-H1 activity was ERK1/2 dependent[24]. As we have shown that Fyn is necessary for mTORC2-mediated RhoA activation in MSCs, and that ERK1/2 is not involved in this cascade[5], we hypothesized that LARG would be responsible for RhoA activation in response to mechanical strain. We also sought to determine which GAP(s) are necessary for balancing RhoA activity by allowing RhoA to cycle back to an inactive state. ARHGAP18, an understudied GAP, controls cell shape, spreading, and motility in fibroblasts and MDA-MB-231 human breast cancer cells[25]. Additionally, knockdown of ARHGAP18 induced sustained RhoA activation, leading us to consider that ARHGAP18 influences RhoA activity in MSCs, thus balancing the activity of Rho GEFs to help “reset” RhoA[25].
In this work, we used murine marrow-derived MSCs to determine the role of Rho GEFs and GAPs on mechanical activation of RhoA and how these GTP exchangers influence RhoA activity to alter MSC fate. We show that mechanical activation of RhoA, and subsequent formation of actin stress fibers requires the GEF LARG. Additionally, mechanical repression of adipogenesis was blunted in the absence of LARG, suggesting that this GEF is a critical GTP exchanger for RhoA-mediated cytoskeletal remodeling in MSCs. We also demonstrated that knockdown of the Rho GAP, ARHGAP18, restricts entry of MSCs into the adipogenic lineage, while promoting osteogenesis. These signals provide reciprocal balance of RhoA activity in MSCs, representing critical control switches to influence lineage decisions. Such factors may be targets for pharmacological or physical therapeutic interventions to enhance bone formation and strength.
Materials and Methods
Reagents
Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Atlanta, GA). Culture media, trypsin-EDTA, antibiotics, and phalloidin-Alexa488 were purchased from Invitrogen (Carlsbad, CA). Insulin, dexamethasone, and indomethacin were purchased from Sigma Aldrich (St. Louis, MO). ROCK inhibitor Y27632 was purchased from Sigma Aldrich (St. Louis, MO). Rhosin inhibitor was purchased from EMD Millipore (Billerica, MA).
siRNA-Mediated Knockdown
mdMSCs were transfected with gene-specific siRNA or control siRNA (20 nM) using PepMute Plus transfection reagent (SignaGen Labs, Rockville, MD). Medium was replaced at 18 hours with IMDM containing FBS (10%, v/v) and penicillin/streptomycin (100 μg/ml). Mechanical strain was applied 72 hours after initial transfection; adipogenic media were added 18 hours after transfection. The following Stealth Select siRNAs (Invitrogen) were used in this study: negative control 5′-CCACUCCCAUUCGGUUACUUAGGAA-3′; LARG 5′-CCAGAUCCCACUUGCUGAUUCUGAA-3′; Vav2 5′-ACAAGCAGCAGCUUCUCUUGUCGUC-3′. Stable knockdown of Arhgap18 was achieved using lentiviral infection of the pLKO.1 plasmid containing shRNA sequence 5′-AATTGATCGATCAAATGGAGG-3′, targeting Arhgap18 (Thermo Scientific). Briefly, lenti virus particles were packaged by the UNC Lenti-shRNA core facility according to the manufacturer’s instructions. Lentiviral particles were added to subconfluent MSC cultures in a 100 cm dish and incubated at 37°C for 48 hrs. Puromycin (20 μg/ml) was then added to select for cells infected with the lentiviral shRNA construct. Media was changed every 48 hrs, with puromycin, until cells grew to 80% confluence. Cells were maintained in IMDM media with puromycin (10 μg/ml); however, fresh media without puromycin was added 24 hours before initiation of experiments.
Antibodies
Anti-RhoA antibody (sc-418) was purchased from Santa Cruz Biotechnology (Dallas, TX). Antibodies recognizing aP2 (XG6174) and alpha-tubulin (PM-7627) were purchased from ProSci, Inc. (Poway, CA). The anti-adiponectin antibody (PA1-054) was from Affinity BioReagents (Rockford, IL). Antibodies recognizing perilipin (#9349) and Pparγ (#2443) were purchased from Cell Signaling (Danvers, MA). The Vav2 antibody (ab52640) was from Abcam (Cambridge, MA). The LARG antibody was a gift from Dr. Kozo Kaibuchi (Nagoya University, Japan), while Dr. Takeshi Senga (Nagoya University, Japan) provided the Arhgap18 antibody.
Cells and Culture Conditions
Marrow-derived mesenchymal stem cells (mdMSCs) were isolated from 8–10 week old C57BL/6 male mice as previously described[26] [27], and maintained in Iscove’s Modified Dulbecco’s Medium (IMDM) with FBS (10%, v/v) and penicillin/streptomycin (100 μg/ml). For experiments, cells were plated at a density of 6,000–10,000 cells/cm2 on collagen-I coated silicone membrane plates (Flexcell International, Hillsborough, NC) and cultured for 2–4 days before beginning experiments. Cells were serum starved overnight in alpha-Minimal Essential Medium (α-MEM) prior to all assays. Adipogenic medium included dexamethasone (0.1 μM), insulin (5 μg/ml) and indomethacin (50 μM). The Y27632 (10 μM) inhibitor was added one hour prior to strain assays.
Mechanical Strain
Uniform equibiaxial strain was applied to mdMSCs plated on six-well Bioflex Collagen-I coated plates using the Flexcell FX-4000 system (Flexcell International). A regimen of 2% strain was delivered at 10 cycles per minute for a total of 100 cycles (10 min) for RhoA activity assays and phosphorylation induction experiments. As development of adipogenesis requires at least 3 days, we subjected MSC during differentiation to 2% strain for 6 hours a day for 3 days. This regimen was used as it was previously reported to be sufficient to restrict adipogenic differentiation of MSCs[28]. Cells used for phosphorylation induction and RhoA activity assays were lysed directly following the 100 cycles of strain, whereas cells stained for actin cytoskeleton were allowed to rest for 3 hrs following 100 cycles of strain to allow for actin stress fiber formation.
Western Blotting
Whole cell lysates were prepared using radio immunoprecipitation assay (RIPA) lysis buffer (150 mM NaCl, 50 mM Tris HCl, 1 mM EGTA, 0.24% sodium deoxycholate,1% Igepal, pH 7.5) containing 25 mM NaF and 2 mM Na3VO4. Aprotinin, leupeptin, pepstatin, and phenylmethylsulfonylfluoride (PMSF) were added before each lysis. Whole cell lysates (20 μg) were separated polyacrylamide gels (7, 9 or 12%) and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with milk (5%, w/v) diluted in TBS-T. Blots were then incubated overnight at 4 C with the appropriate primary antibodies. Blots were washed and incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution) (Cell Signaling) at room temperature (RT) for one hour. Chemiluminescence was detected with ECL plus (Amersham Biosciences, Piscataway, NJ) and developed. Images were acquired with a Hewlett Packard Scanjet scanner, and densitometry was determined using ImageJ software version 1.45s (NIH).
RhoA Assay
Purification of recombinant proteins and construction of the pGEX4T-1 prokaryotic expression constructs containing the Rho-binding domain (RBD) of Rhotekin has been described[29]. Briefly, expression of the fusion proteins in Escherichia coli was induced using isopropyl β-D-1-thiogalactopyranoside (100 μM) for 12–16 hours at RT. Bacterial cells were lysed in lysis buffer containing Tris HCl (50 mM, pH 7.6), NaCl (150 mM), MgCl2 (5 mM), dithiothreitol (1 mM), aprotinin (10 μg/ml), leupeptin (10 μg/ml), and PMSF (1 mM). Recombinant proteins were purified by incubation with glutathione-sepharose 4B beads (GE Healthcare, Piscataway, NJ) at 4 C. Pull down of active RhoA, using glutathione S-transferase-RBD (GST-RBD) beads, was performed as described[30]. mdMSC cells were lysed in buffer containing Tris HCl (50 mM, pH 7.6), NaCl (500 mM), Triton X-100 (1%, v/v), SDS (0.1%, v/v), sodium deoxycholate (0.5%, w/v), MgCl2 (10 mM), orthovanadate (200 μM), and protease inhibitors. Lysates were clarified by centrifugation, equalized for total volume and protein concentration and rotated at 4 C for 30 minutes with 50 μg of purified GST-RBD bound to glutathione-sepharose beads. The bead pellets were washed in lysis buffer, followed by pelleting of the beads by centrifugation, and subsequently processed by SDS-PAGE.
Immunofluorescence
Following strain and/or treatment with pharmacological inhibitors, cells were fixed with paraformaldehyde (4%, v/v) for 20 min, permeabilized with Triton X-100 (0.1%, v/v) for 5 min at RT, and donkey serum (5%, v/v) blocking buffer diluted in TBS-T was added for 30 min to block non-specific epitopes, as previously described[31]. The silicone culture membranes were detached from the BioFlex plates using a scalpel and transferred to wells of six-well plates. Cells were incubated with phalloidin-conjugated Alexa Fluor-488 (Invitrogen) diluted in TBS (1:100) 30 min at RT. Cells were washed and the silicone membranes were set on glass slides, covered, and sealed with mounting medium containing DAPI (Invitrogen). The number of actin stress fibers were quantified in phalloidin stained cells[32]. Briefly, using ImageJ, a line profile was placed across each cell, generating an output corresponding to the peak signal intensity as a function of distance across the cell. Sharp peaks in fluorescence intensity within each line profile represented individual stress fibers. Peak intensity was quantified in 3 different regions per cell, in at least 10 cells per condition.
Statistical Analysis
Statistical variance was expressed as the means ±SE. Statistical significance was evaluated using a natural log transformation of the data to make the distribution more symmetric, followed by analysis using either a t-test or a two-way ANOVA, allowing for unequal variance (Prism GraphPad, La Jolla, CA). Values were considered significant if p was less than or equal to 0.05. Experiments were replicated at least three times to assure reproducibility. Densitometry data, where given, were compiled from at least three separate replicates.
Results
LARG is required for mechanical activation of RhoA in MSCs
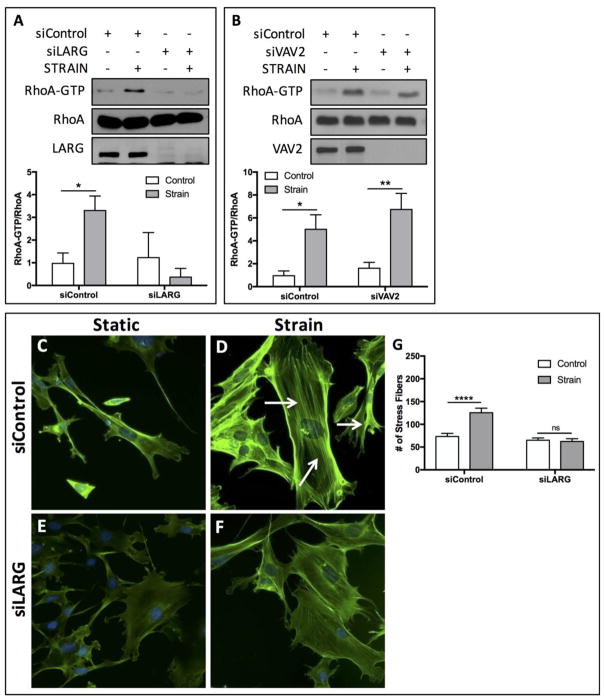
Our prior work demonstrated that 100 cycles of equibiaxial mechanical strain was sufficient to induce actin cytoskeletal stress fiber formation and RhoA activation in MSCs [5, 7, 33]. Here we asked whether the GEF LARG, implicated in tensional activation of RhoA[24] was required for mechanically-induced RhoA activation in MSCs. Following treatment with either a control siRNA or siRNA directed against LARG, MSCs were subject to 100 strain cycles (2%, 10 cycles/min). LARG replete cells demonstrated a significant (p<0.05) increase of 3.32-fold in RhoA activity, as assessed by GTP-bound RhoA (Fig. 1A). Conversely, mechanical strain had no statistically significant effect on RhoA activation following siRNA-mediated silencing of LARG (Fig. 1A). The role of Vav2, another Rho GEF, on mechanical activation of RhoA was also assessed, as previous work showed that stretch-induced RhoA activation in mesangial cells required Vav2[34]. Our results showed that siRNA knockdown of Vav2 in MSCs did not prevent activation of RhoA in response to mechanical strain (Fig. 1B), supporting that LARG is the primary GEF controlling force-mediated RhoA activity in MSCs.
Figure 1.
LARG is required for mechanical activation of RhoA in MSCs. (A) Mechanical treatment of mdMSCs (2% strain, 100 cycles) significantly (p<0.05, n=4) increased RhoA activation (RhoA-GTP). siRNA knockdown (20 nM) of LARG prevented strain-induced RhoA activation. (B) Knockdown of Vav2 did not influence strain-induced activation of RhoA, where mechanical strain significantly increased RhoA activity in the presence (p<0.05, n=3) and absence (p<0.01, n=3) of Vav2. (C–F) Staining of the actin cytoskeleton using alexa 488-conjugated phalloidin showed increased stress fiber formation in strained cells (D) compared to static controls (C). siRNA knockdown of LARG, reduced the stress fiber formation following mechanical strain (F). (G) Quantification of stress fibers confirmed that strain significantly (p<0.0001) increased the number of stress fibers, whereas no significant increase was observed following knockdown of LARG. For actin quantification, at least 15 cells per condition total were analyzed across 3 independent experiments.
To explore the contribution of LARG on actin stress fiber formation, MSCs were strained for 10 minutes followed by a 3 hr rest period prior to staining with Alexafluor-488 conjugated Phalloidin. Compared to static controls (Fig. 1C) 100 cycles of strain was sufficient to enhance actin stress fibers across the cell (Fig. 1D). Treatment with siRNA targeting LARG reduced actin staining in both static (Fig. 1E) and strained cells (Fig. 1F), where depletion of LARG noticeably impaired actin cytoskeletal stress fibers. The number of actin stress fibers was quantified with ImageJ by measuring the peak fluorescent signal intensity in using the line profile tool, where distinct peaks in the fluorescent intensity represented individual stress fibers. Mechanical strain induced a significant (p<0.0001) increase of 1.71-fold in the number of stress fibers (Fig. 1G). Following LARG knockdown, no statistically significant differences in stress fiber numbers were observed (Fig. 1G). These data demonstrate that LARG, but not Vav2, is necessary for strain-induced RhoA activity and actin remodeling in marrow derived MSCs.
LARG knockdown abrogates mechanical repression of adipogenesis through activation of RhoA
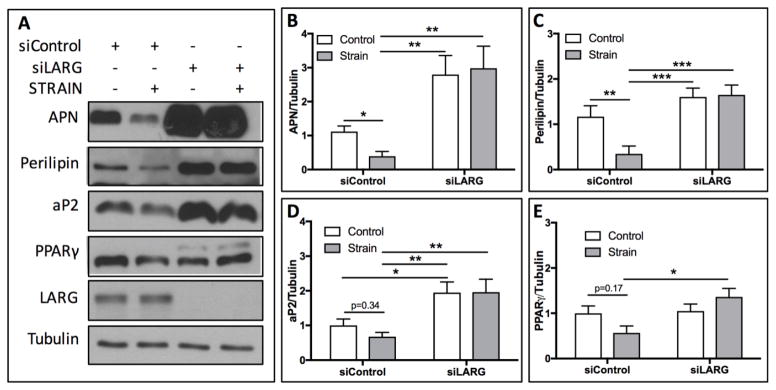
As we have previously shown that mechanical strain represses commitment of MSCs to the adipogenic lineage[6], and RhoA is a critical factor regulating MSC fate[5, 21], we sought to determine whether LARG was necessary for mechanical suppression of adipogenesis. mdMSCs cultured in adipogenic media for 3 days express adipogenic markers aP2, PPARγ, APN, and perilipin (Fig. 2A). Application of mechanical strain (2%, 10 cycles/min) for 6 hours per day for 3 days significantly suppressed expression of adipogenic markers APN by 2.82-fold (Fig. 2B, p<0.05) and perilipin by 3.36-fold (Fig. 2C, p<0.01). Expression of aP2 (Fig. 2D) and PPARγ (Fig. 2E) were decreased by 1.47-fold and 1.76-fold respectively, compared to static controls, yet these values did not reach statistical significance. Cells depleted of LARG, via siRNA knockdown, demonstrated unaltered adipogenic commitment in response to mechanical strain (Fig. 1A–E). Additionally, under basal conditions (non-strained), the absence of LARG resulted in a significant increase in aP2 (1.94-fold, p<0.05, Fig. 2D) expression. Increases in APN (2.8-fold, Fig. 2B), perilipin (1.6-fold, Fig. 2C), and PPARγ (1.04-fold, Fig. 2E) were observed, when compared to non-strained cells treated with a control siRNA, yet statistical significance was not reached for these values. When comparing expression of adipogenic markers in strained cells, with and without LARG, significant increases in APN (7.53-fold, p<0.01), perilipin (4.73-fold, p<0.001), aP2 (2.89-fold, p<0.01), and PPARγ (2.40-fold, p<0.05) were found following LARG knockdown (Fig. 2B–E). These data suggest that LARG is necessary for mechanical strain action to restrict MSC entry into the adipogenic lineage, and under basal conditions, LARG also restricts adipogenic fate of MSCs.
Figure 2.
LARG knockdown abrogates mechanical repression of adipogenesis. (A) Mechanical strain (2%, 6 hr/day, 3 days) of mdMSCs suppressed expression of APN, perilipin, aP2, and PPARγ. (B) Densitometry of Western blot bands demonstrates significant decreases in APN (p<0.05, n=6) and perilipin (C, p<0.01, n=6). While decreased following strain, expression levels of aP2 (p=0.34, n=6), nor PPARγ (p=0.17, n=6) reached statistical significance. Mechanical strain failed to suppress expression of any of these adipogenic markers following knockdown of LARG (B–E).
Knockdown of ARHGAP18 enhances basal activation of RhoA
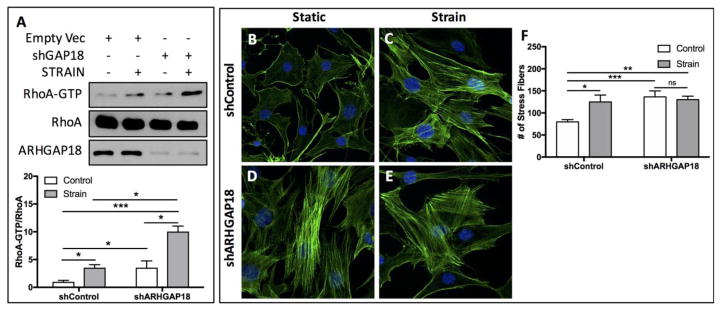
Having determined that the GEF LARG regulates RhoA activity and lineage fate of MSCs, we shifted our attention to Rho GAPs, which inactivate RhoA. Previous work indicated that ARHGAP18 represses RhoA activity leading to impaired cell shape and motility[25]. We considered the contribution of ARHGAP in balancing RhoA activity in response to mechanical strain in MSCs. Stable shRNA-mediated knockdown of ARHGAP18 significantly (p<0.05) increased basal activation of RhoA by 3.54-fold (Fig. 3A), suggesting that ARHGAP18 tonically suppresses RhoA activity. When exposed to mechanical strain, both control MSCs (transfected with an empty vector), as well as cells in which ARHGAP18 was knocked down, showed significant increases in RhoA activity (Fig. 3A). While the activity of RhoA in ARHGAP18 knockdown cells was greater following strain, activity in non-strained cells was also increased compared to cells with normal levels of ARHGAP18 expression. Strain induced a 3.54-fold increase in RhoA activity in shRNA control cells, but only a 2.83-fold increase (p<0.05) was observed following strain in ARHGAP18 knockdown cells. While there was a significant increase (p<0.05) in RhoA activity when comparing cells that were strained with shRNA control and cells strained with an shRNA targeting ARHGAP18, the increase in RhoA activity following strain in cells deficient in ARHGAP18 can be explained by the basal upregulation of active RhoA (Fig. 3A), as knocking down ARHGAP18 increased RhoA activity in non-strained cells to about the equivalent of strained cells containing ARHGAP18. Staining with Alexafluor-488-conjugated phalloidin revealed increased actin stress fiber formation under basal (non-strained) conditions following ARHGAP18 knockdown (Fig. 3D) compared to cells with a control shRNA vector (Fig. 3B), consistent with increased basal RhoA activity in the absence of ARHGAP18. While mechanical strain resulted in enhanced actin stress fiber formation (Fig. 3C), no additional increase was evident in strained MSCs in which ARHGAP18 was knocked down (Fig. 3E). Actin stress fiber numbers were quantified (Fig. 3F), where a significant increase in the number of actin stress was found following mechanical strain (1.56-fold p<0.0001). Consistent with RhoA data, knockdown of ARHGAP18, under non-strained conditions significantly increased actin stress fiber number (1.71-fold, p<0.001). The number of actin stress fibers in cells depleted of ARHGAP18 following strain was 1.63-fold higher (p<0.01) than cells containing shRNA control and not strained; however, no statistically significant change was observed when compared to non-strained ARHGAP18 knockdown cells (Fig. 3F). Thus, ARHGAP18 regulates basal, but not mechanical formation of actin stress fibers by preserving RhoA activation.
Figure 3.
Knockdown of ARHGAP18 enhances basal RhoA activation. (A) Strain significantly (p<0.05, n=3) increased RhoA activation (RhoA-GTP) following strain. Knockdown of ARHGAP18 resulted in increased basal activation of RhoA (p<0.05, n=3). RhoA activation was also significantly increased in the absence of ARHGAP18 following strain (p<0.05, n=3). (B–E) mdMSCs were stained with Alexa488-conjugated phalloidin and dapi following strain (2%, 100 cycles) (C, E) or under static conditions (B, D). Images were captured using a 20x 0.95 plan apo lens. Cells contained either a control shRNA vector (B, C) or an shRNA sequence targeting ARHGAP18 (D, E). (F) Quantification of stress fibers confirmed that strain significantly (p<0.05) increased the number of stress fibers under control conditions. The number of stress fibers was significantly (p<0.001) increased in cell deplete of ARHGAP18 compared to non-strained, ARHGAP18 replete cells. In ARHGAP18 knockdown cells, the number of stress fibers remained approximately equivalent to non-strained, ARHGAP18 knockdown cells, with no significant changes. For actin quantification, at least 15 cells per condition total were analyzed across 3 independent experiments.
Knockdown of ARHGAP18 restricts entry into the adipocyte lineage
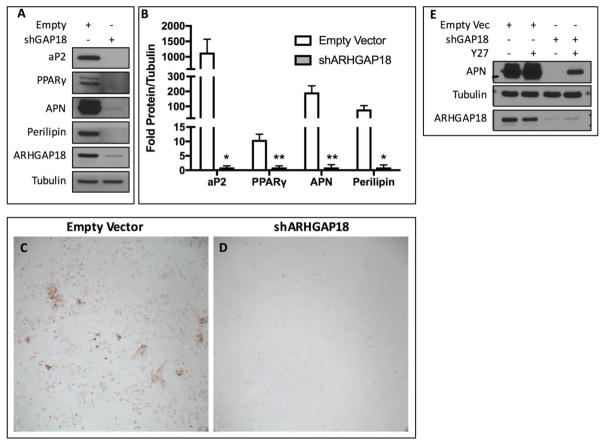
Both matrix stiffness[35] and cellular tension[21] direct MSC fate away from the adipogenic lineage. We hypothesized that enhanced cytoskeletal structure due to the absence of ARHGAP18 would restrict MSC adipogenesis. As expected, control MSCs formed abundant lipid droplets and expressed adipogenic markers (aP2, PPARγ, APN, and perilipin) when cultured for 3 days in adipogenic media (Fig. 4A). In contrast, shRNA knockdown of ARHGAP18 suppressed adipogenesis, significantly reducing expression of adipogenic markers aP2 (1,138.09-fold, p<0.05), PPARγ (10.57-fold, p<0.01), APN (193.74-fold, p<0.01), and perilipin (79.63-fold, p<0.05) compared to control cells in response to adipogenic media (Fig. 4B). Further, ARHGAP18 depleted MSCs had reduced oil-red-O staining (Fig. 4C–D). To determine if inhibition of adipogenesis was due to the effects of ARHGAP18 on RhoA, MSCs were treated with an inhibitor to block the downstream effects of RhoA activation. Addition of the Rho-associated, coiled-coil containing protein kinase 1 (ROCK) inhibitor Y27632[36] partially rescued expression of APN following ARHGAP18 knockdown (Fig. 4E).
Figure 4.
Knockdown of ARHGAP18 suppresses mdMSC adipogenesis. (A) Western blots probing for adipogenic markers aP2, PPARγ, APN, and perilipin following knockdown of ARHGAP18. (B) Densitometry of radiograph bands from Western blots shows significantly decreased expression of aP2 (p<0.05, n=5), PPARγ (p<0.01, n=3), APN (p<0.01, n=5), and perilipin (p<0.05, n=3). (C, D) mdMSC cells stained with oil-red-O, with control shRNA or knockdown of ARHGAP18. (E) Western blot of APN following ARHGAP18 depletion and addition of ROCK inhibitor (Y27632, 10 μM).
Osteogenic potential of mdMSCs is enhanced in the absence of ARHGAP18
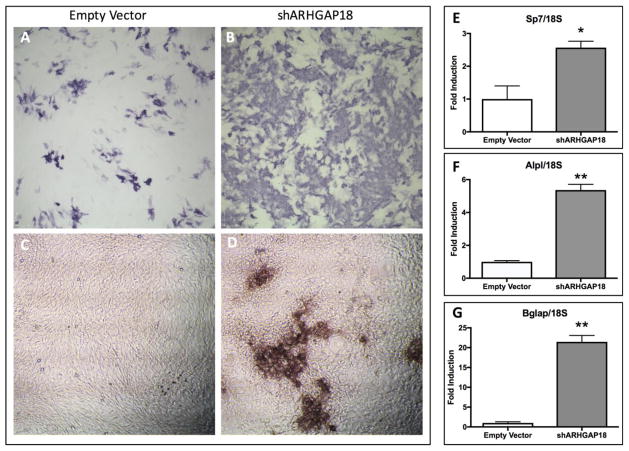
In culture, adipogenic commitment limits the pool of MSC progenitors available for osteogenesis[37]. To determine if ARHGAP18 influences osteogenic differentiation of MSCs, we cultured control and ARHGAP18 knockdown cells in osteogenic media containing ascorbic acid and β-glycerophosphate. An increase in alkaline phosphatase staining in ARHGAP18 knockdown cells was observed compared to controls at 7 days (Fig. 5A–B). Additionally, light microscopy showed formation of prominent osseous nodules in ARHGAP18 knockdown cultures at this time (Fig. 5D), whereas control MSCs lacked nodules at the same time point (Fig. 5C). In a previous study we showed that MSCs require 14 days in osteogenic conditions to generate bone nodules, with subsequent formation of osteocytes[38]. Thus, this observation suggests that ARHGAP18 knockdown accelerates formation of mineralized nodules in culture.
Figure 5.
Osteogenic potential of mdMSCs is enhanced in the absence of ARHGAP18. (A, B) Alkaline phosphatase staining of control (A) and ARHGAP18 deplete (B) MSCs (10x magnification). Light microscopy (10x lens) of control (C) and ARHGAP18 knockdown cells after 7 days in osteogenic media showing presence of mineralized nodules following ARHGAP18 knockdown (D). qPCR analysis of osteogenic genes (E) Sp7 (osterix), (F) Alpl (alkaline phosphatase), and Bglap (osteocalcin) in control and ARHGAP18 knockdown mdMSCs. *p<0.05, **p<0.01, n=3 for all assays.
To further examine the role of ARHGAP18 in osteogenic differentiation, expression of osteogenic genes was assessed by qPCR analysis after 7 days in osteogenic media. Knockdown of ARHGAP18 resulted in a 2.5-fold increase (p<0.05) in osterix (Sp7) expression compared to control MSCs. Additionally, a 5-fold increase (p<0.01) in alkaline phosphatase (Alpl) and a 21-fold increase (p<0.01) in osteocalcin (Bglap) expression was observed following knockdown of ARHGAP18. Taken together with the alkaline phosphatase staining and formation of osseous nodules, these data demonstrate that deficiency of ARHGAP18 is associated with enhanced allocation of MSCs into the osteogenic lineage.
Discussion
Bone health depends on the appropriate allocation of bone marrow mesenchymal progenitors, where increased adipogenic commitment leads to poor bone quality and quantity[39, 40]. Lineage decisions are influenced by cytoskeletal architecture, which is guided by cellular interaction with the static external physical environment[41]. Application of dynamic force, as might be applied to MSCs adherent to bone during exercise, induces activation of the Src-like kinase Fyn, which leads to mTORC2/Akt-mediated RhoA activation[5]. In this way, dynamic mechanical force generates an enhanced actin cytoskeletal structure, which, similarly to its effect in static conditions[21], suppresses adipogenesis. In this study we extend our understanding of the influence of RhoA signaling on MSC lineage fate by showing that the reciprocal control of RhoA is modulated by LARG and ARHGAP18. LARG is necessary for dynamic mechanical activation of RhoA, while ARHGAP18 exerts a tonic control to restrain RhoA. Such regulatory control contributes to lineage biasing, where enhanced RhoA-mediated cytoskeletal structure represses adipogenesis and promotes osteogenesis.
In MSCs, Fyn cooperates with focal adhesion kinase (FAK) to regulate adipogenesis through actin cytoskeletal reorganization[5]. The contribution of FAK to marrow adiposity has been confirmed in vivo, where mice lacking FAK in osterix-expressing osteoblasts have increased accumulation of bone marrow fat with decreased osteogenic differentiation[42]. Our previous work revealed a critical role of Fyn/FAK on mTORC2/Akt signaling resulting in RhoA activation[5]. We now show that force-induced suppression of adipogenesis is abrogated in the absence of LARG, as knockdown of LARG interfered with mechanical repression of adipogenic markers APN, perilipin, and Pparγ. As Fyn/FAK, mTORC2, and Akt act upstream of RhoA[5], these signals likely converge on LARG to regulate the “on-state” of RhoA.
We also observed a significant increase in adipogenic commitment following LARG knockdown in the basal (non-strained) condition, suggesting that LARG is not only important for mechanical regulation of MSC functions, but also restricts entry into the adipogenic lineage. There was no significant decrease in RhoA activity in non-strained cells following LARG knockdown, nor were there significant changes in actin stress fiber numbers. It is possible that LARG depletion alters continual actin remodeling, or might influence other signaling pathways, independent of actin cytoskeletal structure, to modulate cellular differentiation.
RhoGAPs downregulate RhoA activity, cycling it back to the “off-state” [23]. In this study we focused on ARHGAP18, as it was shown to regulate motility, cell shape, and spreading of human MDA-MB-231 breast cancer cells through sustained RhoA activity and enhanced actin structure[25]. Indeed, knockdown of ARHGAP18 in MSCs enhances basal RhoA activity and consequent actin stress fiber formation. Although we hypothesized that knockdown of ARHGAP18 would reset RhoA to be more responsive to mechanical force, we did not find enhanced mechanical activation in its absence. These data suggest that either the mechanical signal does not influence ARHGAP18 activity directly, or in the absence of ARHGAP18, RhoA is maximally activated, interfering with effects of a dynamic strain effect. Our actin stress fiber data support the latter conclusion, in that while ARHGAP18 knockdown, in non-strained cells, increased actin stress fiber number compared to cells containing a control shRNA, no increase in stress fiber number was seen in ARHGAP18 knockdown cells following strain. This suggests that the maximal number of actin stress fibers had been generated, such that strain had no effect to further change cellular stress. Knockdown of ARHGAP18 alone, however, caused a significant reduction in adipogenesis. These data demonstrate that ARHGAP18 plays a previously unrecognized role in regulation of MSC lineage fate, underscoring the importance of cytoskeletal structure in stem cell differentiation. In support of this regulatory function, we show that expression of APN, which was suppressed in ARHGAP18 deficient cells, was rescued by addition of the ROCK inhibitor Y27632.
Cytoskeletal structure is also important for osteoblast differentiation. When cultured in osteogenic media, ARHGAP18 deficient MSCs showed accelerated osteogenesis. Thus, our data suggest that ARHGAP18 regulation of RhoA activity in MSCs may function as a “lineage switch” capable of influencing skeletal homeostasis. As such, ARHGAP18 may represent a novel target to decrease bone marrow adiposity and promote skeletal health.
In summary, lineage decisions of marrow-derived MSCs are influenced by a wide-array of signals, including mechanical input. Application of mechanical force drives cellular rigidity through actin stress fiber formation, leading to adipogenic repression and promotion of osteogenesis. We here have identified two molecules that reciprocally regulate the activity of RhoA to influence MSC lineage. On one hand, LARG restricts adipogenesis and is necessary for mechanical and basal activation of RhoA, while ARHGAP18 promotes adipogenesis and suppresses osteogenesis via inhibition of RhoA activity. In conclusion, the balance of RhoA activity through GEF and GAP action has a role in determining the lineage fate of MSC progenitors. Targeting LARG or ARHGAP18 through physiological loading regimens or by pharmacological means may prove a useful strategy to restrain adipose formation and promote skeletal integrity.
Highlights.
LARG, a Rho GEF, is necessary for mechanical activation of RhoA.
Knockdown of LARG prevents mechanical suppression of adipogenesis.
ARHGAP18, a Rho GAP, restricts RhoA to restrain entry into the osteogenic lineage.
ARHGAP18 knockdown increases actin fibers and decreases adipogenic differentiation.
Acknowledgments
Funding support: This study was supported by AR056655, AR064133, AR069943, GM029860, P20GM109095, AR062097
Footnotes
Author Contributions:
William Thompson: concept/design, collection/assembly of data, data analysis/interpretation, manuscript writing, final approval of manuscript
Sherwin Yen: concept/design, collection/assembly of data, data analysis/interpretation, final approval of manuscript
Gunes Uzer: concept/design, collection/assembly of data, data analysis/interpretation, final approval of manuscript
Zhihui Xie: collection/assembly of data
Buer Sen: data analysis/interpretation, final approval of manuscript
Maya Styner: data analysis/interpretation, final approval of manuscript
Keith Burridge: concept/design, data analysis/interpretation, final approval of manuscript
Janet Rubin: concept/design, financial support, data analysis/interpretation, manuscript writing, final approval of manuscript
Conflict of Interest: All authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Abreu MR, Wesselly M, Chung CB, Resnick D. Bone marrow MR imaging findings in disuse osteoporosis. Skeletal Radiol. 2011;40(5):571–5. doi: 10.1007/s00256-010-1042-x. [DOI] [PubMed] [Google Scholar]
- 2.David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148(5):2553–62. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 3.Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, Case N, Xie Z, Sen B, Romaine A, Pagnotti GM, Rubin CT, Styner MA, Horowitz MC, Rubin J. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64(0):39–46. doi: 10.1016/j.bone.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, Sen B, Xie Z, Horowitz MC, Styner MA, Rubin C, Rubin J. Exercise Regulation of Marrow Fat in the Setting of PPARgamma Agonist Treatment in Female C57BL/6 Mice. Endocrinology. 2015;156(8):2753–61. doi: 10.1210/en.2015-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson WR, Guilluy C, Xie Z, Sen B, Brobst KE, Yen SS, Uzer G, Styner M, Case N, Burridge K, Rubin J. Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem cells. 2013;31(11):2528–37. doi: 10.1002/stem.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen B, Xie ZH, Case N, Ma MY, Rubin C, Rubin J. Mechanical Strain Inhibits Adipogenesis in Mesenchymal Stem Cells by Stimulating a Durable beta-Catenin Signal. Endocrinology. 2008;149(12):6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen B, Guilluy C, Xie Z, Case N, Styner M, Thomas J, Oguz I, Rubin C, Burridge K, Rubin J. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem cells. 2011;29(11):1829–36. doi: 10.1002/stem.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warden SJ, Thompson WR. Become one with the force: optimising mechanotherapy through an understanding of mechanobiology. Br J Sports Med. 2017;51(13):989–990. doi: 10.1136/bjsports-2017-097634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson WR, Scott A, Loghmani MT, Ward SR, Warden SJ. Understanding Mechanobiology: Physical Therapists as a Force in Mechanotherapy and Musculoskeletal Regenerative Rehabilitation. Phys Ther. 2016;96(4):560–9. doi: 10.2522/ptj.20150224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uzer G, Fuchs RK, Rubin J, Thompson WR. Concise Review: Plasma and Nuclear Membranes Convey Mechanical Information to Regulate Mesenchymal Stem Cell Lineage. Stem cells. 2016;34(6):1455–63. doi: 10.1002/stem.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503(2):179–93. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokol SY. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development. 2011;138(20):4341–50. doi: 10.1242/dev.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical Loading Regulates NFATc1 and β-Catenin Signaling through a GSK3β Control Node. Journal of Biological Chemistry. 2009;284(50):34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Case N, Thomas J, Sen B, Styner M, Xie Z, Galior K, Rubin J. Mechanical regulation of glycogen synthase kinase 3beta (GSK3beta) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein. The Journal of biological chemistry. 2011;286(45):39450–6. doi: 10.1074/jbc.M111.265330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 16.Sen B, Xie Z, Case N, Thompson WR, Uzer G, Styner M, Rubin J. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(1):78–89. doi: 10.1002/jbmr.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen B, Xie Z, Case N, Styner M, Rubin CT, Rubin J. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. Journal of biomechanics. 2011;44(4):593–9. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josselyn SA, Frankland PW. mTORC2: actin on your memory. Nat Neurosci. 2013;16(4):379–80. doi: 10.1038/nn.3362. [DOI] [PubMed] [Google Scholar]
- 19.Mok KW, Mruk DD, Lee WM, Cheng CY. Rictor/mTORC2 regulates blood-testis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(3):1137–52. doi: 10.1096/fj.12-212977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter CL. Actin cytoskeleton and cell signaling. Crit Care Med. 2000;28(4 Suppl):N94–9. doi: 10.1097/00003246-200004001-00011. [DOI] [PubMed] [Google Scholar]
- 21.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Developmental Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 22.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 23.Lawson CD, Burridge K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases. 2014;5:e27958. doi: 10.4161/sgtp.27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13(6):722–7. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda M, Hasegawa H, Hyodo T, Ito S, Asano E, Yuang H, Funasaka K, Shimokata K, Hasegawa Y, Hamaguchi M, Senga T. ARHGAP18, a GTPase-activating protein for RhoA, controls cell shape, spreading, and motility. Mol Biol Cell. 2011;22(20):3840–52. doi: 10.1091/mbc.E11-04-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. 2004 doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 27.Case N, Xie Z, Sen B, Styner M, Zou M, O’Conor C, Horowitz M, Rubin J. Mechanical activation of beta-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells. J Orthop Res. 2010;28(11):1531–8. doi: 10.1002/jor.21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Case N, Xie Z, Sen B, Styner M, Zou M, O’Conor C, Horowitz M, Rubin J. Mechanical activation of β-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells. Journal of Orthopaedic Research. 2010;28(11):1531–1538. doi: 10.1002/jor.21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu BP, Burridge K. Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not beta1 integrins. Mol Cell Biol. 2000;20(19):7160–9. doi: 10.1128/mcb.20.19.7160-7169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12(9):2711–20. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson WR, Majid AS, Czymmek KJ, Ruff AL, García J, Duncan RL, Farach-Carson MC. Association of the α2Δ1 subunit with Cav3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes. Journal of Bone and Mineral Research. 2011;26(9):2125–2139. doi: 10.1002/jbmr.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acharya PS, Majumdar S, Jacob M, Hayden J, Mrass P, Weninger W, Assoian RK, Pure E. Fibroblast migration is mediated by CD44-dependent TGF beta activation. Journal of cell science. 2008;121(Pt 9):1393–402. doi: 10.1242/jcs.021683. [DOI] [PubMed] [Google Scholar]
- 33.Sen B, Xie Z, Case N, Thompson WR, Uzer G, Styner M, Rubin J. mTORC2 Regulates Mechanically Induced Cytoskeletal Reorganization and Lineage Selection in Marrow-Derived Mesenchymal Stem Cells. Journal of Bone and Mineral Research. 2014;29(1):78–89. doi: 10.1002/jbmr.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng F, Zhang B, Ingram AJ, Gao B, Zhang Y, Krepinsky JC. Mechanical stretch-induced RhoA activation is mediated by the RhoGEF Vav2 in mesangial cells. Cell Signal. 2010;22(1):34–40. doi: 10.1016/j.cellsig.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 36.Fujisawa K, Madaule P, Ishizaki T, Watanabe G, Bito H, Saito Y, Hall A, Narumiya S. Different regions of Rho determine Rho-selective binding of different classes of Rho target molecules. The Journal of biological chemistry. 1998;273(30):18943–9. doi: 10.1074/jbc.273.30.18943. [DOI] [PubMed] [Google Scholar]
- 37.Veldhuis-Vlug AG, Rosen CJ. Mechanisms of marrow adiposity and its implications for skeletal health. Metabolism. 2017;67:106–114. doi: 10.1016/j.metabol.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson WR, Uzer G, Brobst KE, Xie Z, Sen B, Yen SS, Styner M, Rubin J. Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model. Scientific reports. 2015;5:11049. doi: 10.1038/srep11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113(6):846–55. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho SW, Yang JY, Her SJ, Choi HJ, Jung JY, Sun HJ, An JH, Cho HY, Kim SW, Park KS, Kim SY, Baek WY, Kim JE, Yim M, Shin CS. Osteoblast-targeted overexpression of PPARgamma inhibited bone mass gain in male mice and accelerated ovariectomy-induced bone loss in female mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(8):1939–52. doi: 10.1002/jbmr.366. [DOI] [PubMed] [Google Scholar]
- 41.Mathieu PS, Loboa EG. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev. 2012;18(6):436–44. doi: 10.1089/ten.teb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun C, Yuan H, Wang L, Wei X, Williams L, Krebsbach PH, Guan JL, Liu F. FAK Promotes Osteoblast Progenitor Cell Proliferation and Differentiation by Enhancing Wnt Signaling. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31(12):2227–2238. doi: 10.1002/jbmr.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]