Abstract
Factors influencing the development of alcohol use disorder (AUD) are complex and heterogeneous. While animal models have been crucial to identifying actions of alcohol on neural cells, human-derived in vitro systems that reflect an individual’s genetic background hold promise in furthering our understanding of the molecular and functional effects of alcohol exposure and the pathophysiology of AUD. In this report, we utilized induced pluripotent stem cell (iPSCs)-derived neural cell cultures obtained from healthy individuals (CTLs) and those with alcohol dependence (ADs) to (1) examine the effect of 21-day alcohol exposure on mRNA expression of three genes encoding GABAA receptor subunits (GABRA1, GABRG2, and GABRD) using quantitative PCR and (2) examine the effect of acute and chronic alcohol exposure on GABA-evoked currents using whole cell patch clamp electrophysiology. iPSCs from CTLs and ADs were differentiated into neural cultures enriched for forebrain-type excitatory glutamate neurons. Following 21-day alcohol exposure, significant treatment effects were observed in GABRA1, GABRG2, and GABRD mRNA expression. A modestly significant interaction between treatment and donor phenotype was observed for GABRD, which was increased in cell cultures derived from ADs. No effect of acute or chronic alcohol was observed on GABA-evoked currents in neurons from either CTLs or ADs. This work extends findings examining the effects of alcohol on the GABAA receptor in human cell in vitro model systems.
Keywords: Alcohol use disorder, iPSC, Induced pluripotent stem cells, GABA receptor, Gene expression, Electrophysiology
Introduction
Alcohol use disorder (AUD) is a prevalent condition which affects nearly 14% of the U.S. population during a given year (Grant et al. 2015). Inhibitory gamma aminobutyric acid type-A (GABAA) ionotropic receptors have been implicated in the behavioral effects of alcohol and the pathophysiology of alcohol use disorder (Enoch 2008). GABAA receptors are pentameric structures composed of various combinations of 2α, 2β and 1 non-α/β subunit (19 GABAA subunits have been identified α1-6, β1-3, γ1-3, δ, ε, θ, π, and ρ1-3) that surround a chloride-permeable pore. The subunit stoichiometry of receptors dictates their functional and pharmacological properties (Olsen and Sieghart 2009). Interactions between alcohol and GABAA receptors were first suggested by similarities between the behavioral effects of alcohol and the GABAA allosteric modulators benzodiazepines and barbiturates, the cross tolerance that develops between alcohol and these drugs, and findings in rodent models indicating that the behavioral effects of acute alcohol intoxication can be modified by co-administration of various agonists or antagonists of the GABAA receptor (for detailed reviews, see (Grobin et al. 1998, Weiner and Valenzuela 2006, Kumar et al. 2009).
Electrophysiological recordings from rodents provides evidence for potentiating effects of alcohol on postsynaptic GABAA receptor activity in neurons located in the cortex and hippocampus (Aguayo 1990, Weiner et al. 1994), amygdala (Nie et al. 2004), nucleus accumbens (Nie et al. 2000), and the retina (Yeh and Kolb 1997), although subsequent research has yielded variable findings including no effect (Criswell et al. 2003) or an attenuating effect (Xie et al. 2013) of alcohol, suggesting that alcohol’s actions at these receptors are complex. The GABAA receptor’s sensitivity to alcohol may be dependent on subunit stoichiometry (Sundstrom-Poromaa et al. 2002, Wei et al. 2004, Wallner et al. 2006), and molecular adaptations that regulate subunit expression in response to prolonged alcohol exposure are thought to contribute to alcohol tolerance and withdrawal. In rodent models, chronic alcohol exposure produced changes in GABAA subunit gene expression that varied with the length of alcohol exposure, duration of the withdrawal period, and the brain region examined (Mhatre and Ticku 1992, Mhatre and Ticku 1994, Devaud et al. 1995, Kumar et al. 2009). Because the subunit composition of GABAA receptors influence their functional and pharmacological properties (Olsen and Sieghart 2009), the changes in subunit expression following chronic alcohol exposure are thought to restore excitatory/inhibitory balance. In in vivo and in vitro rodent studies, chronic alcohol exposure increased the expression of the GABAA α4 subunit and decreased the expression of the α1 subunit (Devaud et al. 1995, Cagetti et al. 2003), a switch that reduces the chloride current passing through exogenously expressed GABAA receptors in mammalian cells (Picton and Fisher 2007), consistent with a compensatory response to alcohol’s potentiation of inhibitory transmission. Studies examining post mortem human brain samples from alcoholics and controls have reported differences in expression of the γ2 (Enoch et al. 2012) and δ subunits (Bhandage et al. 2014), although observed differences in expression may be limited to specific brain regions (Jin et al. 2012, Jin et al. 2014).
Induced pluripotent stem cell (iPSC) technologies (Takahashi et al. 2007) allow for the in vitro examination of human neural cultures derived from characterized donor subjects to explore molecular phenotypes related to disease. Biological mechanisms underlying psychiatric disorders including addiction are complex and heterogeneous. Our understanding of these disorders could benefit from an examination of human neural cultures in vitro. To date, only a few studies have utilized human iPSCs in addiction research, including reports examining opioid (Sheng et al. 2016), nicotine (Oni et al. 2016), and alcohol use disorder (Lieberman et al. 2012, Lieberman et al. 2015). In the current report, we generated iPSC-derived neural cultures from a total of 24 donor subjects (11 controls and 13 alcoholics) and examined the effects of acute and chronic alcohol exposure on the expression and function of the inhibitory ionotropic GABAA receptor, a well-characterized target of alcohol.
Materials and Methods
iPSC Derivation and Culture
iPSCs were generated from fibroblasts obtained using a skin punch biopsies of the inner, upper arm from 24 participants enrolled in studies at UCONN Health (UC, Farmington, CT). Fibroblasts from 11 control subjects (CTLs) were generated from non-alcoholic participants enrolled in a study examining the subjective effects of acute alcohol intoxication (Covault et al. 2014, Milivojevic et al. 2014). Fibroblasts from 13 individuals with a DSM-IV diagnosis of alcohol dependence (ADs) were generated from participants enrolled in a pharmacological treatment study (Kranzler et al. 2014). The average age of CTLs was 30.5 years while that of ADs was 46.1 years. All CTLs were male, while the ADs included 10 males and 3 females. The control sample was limited to males due to restrictions of enrollment in the clinical study the fibroblast samples were obtained (Covault et al. 2014). Biopsy samples were minced and cultured in Dulbecco’s modified eagles medium (DMEM, Thermo Fisher Scientific) supplemented with 20% fetal bovine serum (FBS, Thermo Fisher Scientific), 1x non-essential amino acids (Thermo Fisher Scientific) and 1x penicillin/streptomycin (Thermo Fisher Scientific). Fibroblast cultures were expanded and passaged using trypsin (Thermo Fisher Scientific) prior to being frozen or sent for reprogramming.
The UC Stem Cell Core (Farmington, CT) reprogrammed fibroblasts to pluripotency using a retrovirus to express five factors (OCT4, SOX2, KLF4, c-MYC, and LIN28) or a sendai virus to express four factors (OCT4, SOX2, KLF4, and c-MYC), reflecting a change in reprogramming methodology over the course of the project. Two to four weeks after viral transduction, multiple pluripotent stem cell colonies for each subject were selected and expanded as individual iPSC lines. Expression of pluripotency markers by iPSC cells was verified by immunocytochemistry for SSEA-3/4 and NANOG by the UC Stem Cell Core. The total sample for the current experiments consisted of 35 iPSC lines generated from the 24 subjects, with 2 (or 3) clones examined from 10 subjects (6 CTLs and 4 ADs) and 1 clone examined from 14 subjects (5 CTLs and 9 ADs). In total, 5 of the 35 lines examined in this report were from female donors.
As previously described (Lieberman et al. 2012), iPSCs were cultured on a feeder layer of irradiated mouse embryo fibroblasts using human embryonic stem cell media containing DMEM with F12 (DMEM/F12, 1:1 ratio, Thermo Fisher Scientific) supplemented with 20% Knockout Serum Replacer (Thermo Fisher Scientific), 1x non-essential amino acids, 1 mM L-glutamine (Thermo Fisher Scientific), 0.1 mM β-mercaptoethanol (MP Biomedicals), and 4 ng/mL of basic fibroblast growth factor (bFGF, Millipore). iPSCs were monitored daily and colonies exhibiting spontaneous differentiation were manually removed. Media was fully replaced daily and cells were cultured for 7 days, or to confluency, before being passaged using 1 mg/mL Dispase (Thermo Fisher Scientific) in DMEM/F12.
Neural Differentiation
iPSCs were differentiated into neural cell cultures as previously described (Lieberman et al. 2012) using an established protocol developed by the WiCell Institute for the differentiation of human embryonic stem cells into neural cells of a forebrain lineage (#SOP-CH-207, REV A, www.wicell.org, Madison, WI). In the absence of specific neuronal lineage morphogens the protocol results in forebrain neurons containing both glutamatergic and GABAergic neurons in an approximately 3:1 ratio as well as astrocytes (Fink et al., 2017). Mature neurons contain focal clusters of synaptic proteins and exhibit spontaneous excitatory and inhibitory currents. Briefly, we used an “Embryoid Body” (EB)-based protocol wherein iPSC colonies are removed from the feeder layer substrate and cultured in suspension before going through a neural induction phase to generate primitive neuroepithelial cells. Following 3 weeks of culture in neural induction media containing 1x N2 supplement and 2 μg/mL heparin (Sigma-Aldrich), cells were dissociated and cultured in 24-well plates on 0.1mg/mL polyornithine and Matrigel (BD Biosciences, Bedford, MA) coated glass coverslips in neural differentiation media containing the neural growth factors 1x B27 supplement (Thermo Fisher Scientific), 1μg/mL laminin (Sigma-Aldrich), and 10ng/mL of each brain-derived neurotrophic factor (BDNF, Peprotech, Rocky Hill, NJ), glial-derived neurotrophic factor (GDNF, Peprotech), and insulin-like growth factor 1 (IGF-1, Peprotech). All cells were incubated at 37° in 5% CO2.
Immunocytochemistry
Twelve weeks after being plated onto glass coverslips, neural cell lines from CTLs and ADs were fixed using 4% paraformaldehyde in PBS for 20 min at room temperature and permeabilized using 0.2% triton X-100 (Sigma-Aldrich) in PBS for 10 min. Following a 1-hr block using 5% donkey serum (Jackson ImmunoResearch, West Grove, PA), cultures were incubated for 24–48 hours at 4° with the following primary antibodies diluted in 5% donkey serum in PBS: mouse anti-GFAP (1:500, Millipore, Brillerica, CA), rabbit anti-MAP2 (1:500, Millipore), or rabbit anti-TBR1 (a forebrain glutamatergic neuron marker; 1:1000, ProteinTech Group, Chicago, IL, incubation included 0.1% triton X-100). Cells were then incubated at room temperature for 2-hours in donkey anti-mouse alexa fluor 594 (1:1000, Thermo Fisher Scientific) and donkey anti-rabbit alexa fluor 488 (1:1000, Thermo Fisher Scientific) secondary antibodies diluted in 3% donkey serum in PBS for visualization. TBR1+ cells as a percentage of the total cell population were quantified from 6 control and 6 alcoholic neural cell lines (derived from 5 control and 6 alcoholic donor subjects).
Gene expression of GABAA receptor subunits
Twelve-week-old neural cultures were used to examine GABAA subunit mRNA expression following alcohol exposure. In total, 22 neural cell lines derived from 15 donor subjects (13 lines from 8 CTLs, 9 lines from 7 ADs) were used. We cultured 3–4 coverslips per subject, per condition in either neural differentiation media (sham condition) or neural differentiation media supplemented with 50 mM alcohol for 21 days. Neural differentiation media with or without alcohol was replaced daily. Evaporation results in ~18 mM alcohol remaining at 24 hours after treatment (Lieberman et al. 2012). Wells fed with alcohol-containing media were separated in different culture plates to eliminate transfer of alcohol vapor into the sham condition wells. RNA was extracted upon completion of the three-week alcohol exposure using TRIzol reagent (Thermo Fisher Scientific) following the manufacturer’s instructions. RNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Fischer Scientific, Pittsburgh, PA) and cDNA was synthesized from 2 μg RNA using a High Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific).
cDNA was analyzed by quantitative real-time polymerase chain reaction using an Applied Biosystems 7500 instrument (Thermo Fisher Scientific) and TaqMan Assay On Demand (Thermo Fisher Scientific) FAM-labeled probe and primer sets for: GABRA1 (hs00168058_m1), GABRG2 (hs00158093_m1), and GABRD (hs00181309_m1). Expression of these genes was normalized to the VIC-labeled housekeeping gene GUSB (4326320E), which we used due to its moderate abundance in our culture system and our prior demonstration of its reliability in studies of alcohol and genetic variation in iPSC-derived neural cells (Lieberman et al. 2012, Lieberman et al. 2015). cDNA synthesized from RNA extracted from each culture well was assayed in triplicate 20 μL reactions using Gene Expression Master Mix (Thermo Fisher Scientific) per the manufacturer’s protocol.
PCR cycles were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec. A standard curve consisting of a 4-level serial dilution of 100%, 50%, 25%, and 12.5% cDNA from 20-week old neural cells differentiated from one subject was added to each plate to determine the relative mRNA expression among all subjects and across different qPCR plates. Data are displayed as mRNA abundance normalized to the housekeeping gene GUSB where a unit of 1 is equivalent to the abundance of the target gene relative to GUSB in the reference RNA sample.
Electrophysiology
Whole cell patch-clamp electrophysiology was performed on mature neurons (20–36 weeks old) derived from 6 CTL and 7 AD cell lines using previously described techniques (Lemtiri-Chlieh and Levine 2010). Neurons were selected for recording based on morphology, including pyramidal-shaped soma and presence of neurites. Artificial cerebrospinal fluid (aCSF) containing 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 1 mM MgCl2-6H2O, 25 mM NaHCO3, 2 mM CaCl2, and 25 mM dextrose was perfused through the recording chamber at 1–2 ml/min at room temperature. A high chloride internal recording solution was used containing 130 mM KCl, 10 mM HEPES, 10 mM phosphocreatine, 1 mM EGTA, 0.1 mM CaCl2, 1.5 mM MgCl2, 4 mM Na2-ATP, and 0.3 mM Na-GTP (pH 7.3), which allowed for GABA-evoked currents to be examined at a resting membrane potential of −70 mV. Upon break-in, patched cells were confirmed as neurons by the presence of voltage-gated sodium and potassium currents upon depolarizing voltage steps, ability of cells to fire an action potential upon depolarizing current injections, and noted for their resting membrane potential by injection with 0 current, which was corrected post hoc for liquid junction potential. Inward and outward currents were elicited in voltage clamp mode by applying 300-ms steps from −100 mV to +40 mV in 10 mV increments with the membrane potential held at −70 mV. Action potentials were evoked in current clamp mode at ~−70 mV by applying 500-ms duration current steps from −20 pA to +40 pA in 5 pA intervals. Only cells that could fire a mature action potential (either a single spike or a train of action potentials) were analyzed.
To examine the effect of acute alcohol exposure on GABAA-receptor-mediated chloride currents in neurons from CTLs and ADs, a micropipette attached to a positive-pressure applying Picospritzer (Parker Hannifin Corp) was filled with aCSF containing 50 μM GABA and placed ≈20–30 μm away from the patched neuron. GABAA-mediated currents were examined in voltage clamp mode with the cell held at −70 mV. Currents were evoked every 30 sec by a 50–200 mS focal GABA application at 5–10 PSI. Following a 5-min baseline recording in normal aCSF, the perfusion was switched to an aCSF containing 50 mM alcohol for 15 minutes, following which a normal aCSF was perfused for a 15-min washout period.
The peak of GABA-evoked responses was used to examine the effect of chronic 50 mM alcohol exposure (9–21 days) on GABA-mediated current in neurons from CTLs and ADs. Sham- or alcohol-treated coverslips were removed from the incubator, placed in the recording chamber containing oxygenated aCSF, and neurons were patched and validated by the presence of voltage-gated sodium and potassium channels and the ability to fire an action potential. Following a 1–2 min baseline recording in normal aCSF in voltage clamp mode at −70 mV, GABA-mediated current was examined by bath application of aCSF containing 10 μM GABA. The change in current was monitored and the perfusion tube was switched back to a normal aCSF upon plateau and slight reversal of the GABA-evoked current, which occurred after ≈ 30 seconds. The maximum value of the GABA-evoked current was normalized to membrane capacitance (pF) to control for differences in cell size.
All electrophysiological recordings were performed using a HEKA EPC9 amplifier and PatchMaster software (version 2×67). Analysis and quantification of peak GABA-mediated currents was performed using Axon Clampfit software (version 10.3.1.4).
Statistical Analysis
All statistical analyses were performed using GraphPad Prism software (V5.0f for Mac, GraphPad Software, www.graphpad.com). Student’s t-tests were used to compare basal differences between neural cells derived from controls and alcoholics. A two-way repeated measures ANOVA containing donor status (i.e. control or alcoholic) and alcohol treatment as factors was utilized to examine the effect of 21-day alcohol exposure on GABAA subunit gene expression and the effect of acute alcohol exposure on GABA-evoked currents. The electrophysiology analysis of puff-applied GABA currents compared a 5-minute baseline recording to the last 5-minutes of acute alcohol exposure. A regular two-way ANOVA containing donor status and alcohol treatment was used to examine the effect of chronic alcohol exposure on bath-applied GABA-evoked currents. Statistical significance was defined as p < 0.05.
Results
Chronic alcohol exposure regulates GABAA subunit gene expression
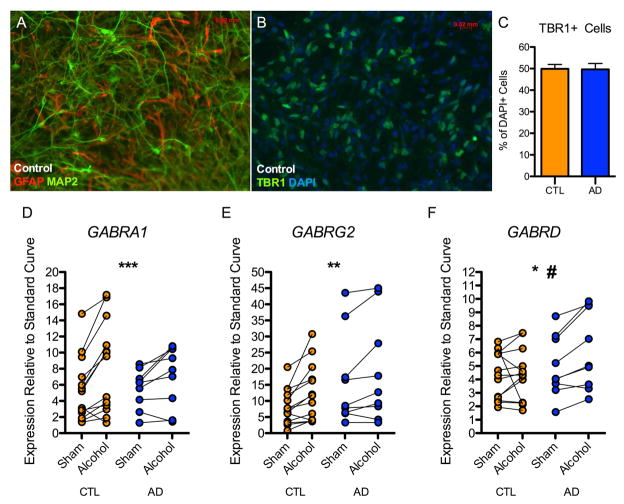
Twelve-week-old iPSC derived neural cultures contained a mixture of MAP2-postive neurons and GFAP-positive astrocytes (Figure 1A), with no overall morphological differences observed between neural cultures derived from CTLs and ADs. About half of the total cells in these cultures expressed TBR1, a transcription factor marking excitatory forebrain-type glutamate neurons. The percentage of TBR1+ cells did not differ between 6 CTL and 6 AD neural cell lines (p = 0.94) (Figure 1B–C). Neural cultures generated by this protocol also include GAD67 positive GABA neurons (~ 20% of cells; Fink et al., 2017)
Figure 1. Effect of 21-day alcohol exposure on GABAA subunit mRNA expression.
12–14 week old neural cultures derived from controls and alcoholics were characterized by immunocytochemistry. (A) Representative image from a control subject indicating iPSCs differentiate into mixed neural cultures containing MAP2-postive neurons and GFAP-positive astrocytes. (B) Representative image from a control subject indicating that cultures are enriched for TBR1+ forebrain-type glutamate neurons. (C) No difference was observed in the number of TBR1+ neurons between neural cultures derived from controls and alcoholics. 10,255 DAPI+ cells derived from 6 control and 6 alcoholic neural cell lines were analyzed. (D, E, F) 12-week old neural cell lines (13 control and 9 alcoholic) were treated daily with neural media supplemented with 50 mM alcohol. (D) A significant effect of alcohol treatment was observed for GABRA1 gene expression. (E) A significant effect of alcohol treatment was observed for GABRG2 gene expression. (F) A significant effect of alcohol treatment was observed for GABRD gene expression. A modestly significant interaction between donor status (alcoholic vs. control) and alcohol treatment was observed. (Two-way repeated measures ANOVA: *p < 0.05, **p < 0.01, ***p < 0.001 for alcohol treatment; #p = 0.054 for interaction between donor status and alcohol treatment)
Neural cultures derived from 13 CTL and 9 AD neural cell lines were exposed to neural differentiation media or media containing 50 mM alcohol for 21 days, with media replaced daily. We examined mRNA expression of GABAA subunit-encoding genes GABRA1, GABRG2, and GABRD. Two-way repeated measures ANOVA revealed significant effects of alcohol treatment on GABRA1 [F(1,40)= 19.9, p < 0.001] GABRG2 [F(1,40)= 13.8, p < 0.01], and GABRD [F(1,40)= 4.6, p < 0.05] gene expression (Figure 1D–F). No significant main effect of donor status was observed for any of the three genes (p’s > 0.05). There was no significant interaction between alcohol treatment and donor status for GABRA1 or GABRG1 (p’s > 0.05), while a modestly significant interaction was observed for GABRD [F(1,40) = 4.2, p = 0.054], such that average gene expression was unchanged in control-derived cultures and increased in alcoholic-derived neural cultures by 24%. The 3- to 8-fold range in basal expression among iPSC lines (Figure 1D–F) could reflect different rates of neural maturation of individual lines and/or individual differences in donor genome between lines. The variation in expression change between lines after chronic alcohol exposure is more modest and may reflect genomic variation, as it represents a within-subject (culture) difference. The cell lines used for gene expression analysis included independently generated clones derived from 5 CTL and 2 AD donors. Examination of the concordance for the direction of change in RNA following alcohol exposure (increase or decrease relative to sham) for the 7 pairs of replicate clones derived from individual donors for the three GABAA subunit genes showed directional concordance for 16 and non-concordance for 5 clones, suggesting that individual genomic variation between subjects may contribute to observable variation in gene expression response. Of the 16 concordant expression changes among pairs, 15 were upregulated and 1 was downregulated.
Acute or chronic alcohol exposure does not alter GABA-evoked current
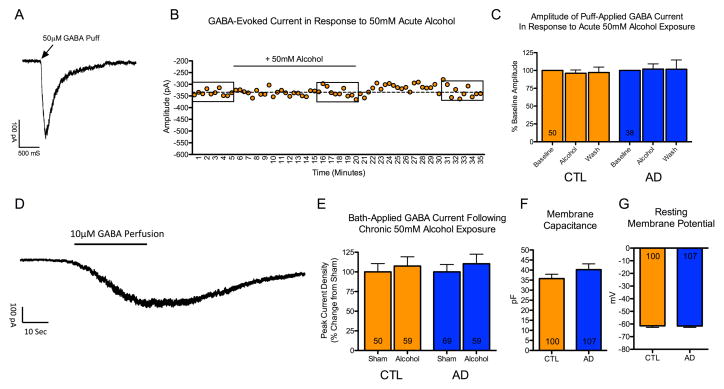
To examine the effects of acute alcohol exposure on the amplitude of GABA-mediated currents, responses were evoked by pressure application of 50 μM GABA in mature (20–33 week old) neurons derived from CTLs and ADs (Figure 2A). These responses could be consistently evoked every 30 sec over a 35-min recording period (Figure 2B). The acute effect of alcohol was examined in a total of 88 neurons derived from 6 CTL and 7 AD lines (average of ≈7 cells per subject). There was no significant effect of 15-minute bath perfusion of 50 mM alcohol on the amplitude of GABA-evoked response (p = 0.81), and there was no significant interaction between alcohol treatment and donor status (p = 0.48) (Figure 2C).
Figure 2. Effect of acute and chronic alcohol on GABA-evoked currents.
Mature neurons (22–36 weeks post plating) derived from 6 control and 7 alcoholic lines were used for electrophysiological recordings. (A) Example trace of a GABA-evoked current in response to brief pressure application of 50 μM GABA. (B) Example recording of a neuron from a control subject. Each dot represents a GABA-evoked response, which was evoked every 30-seconds. Following a 5-minute baseline recording, aCSF containing 50 mM alcohol was perfused for a total of 15 minutes, which was subsequently washed out for an additional 15 minutes. Boxes indicate responses within 5-minute bins that were averaged for analysis. (C) No significant effect of acute alcohol was observed on the amplitude of GABA-evoked responses in neurons derived from 6 control or 7 alcoholic iPSC lines. Washout contains data from a subset of 46 control neurons and 32 alcoholic neurons. (D) Example trace of a GABA response in a control neuron evoked by bath perfusion of aCSF supplemented with 10 μM GABA. Bath perfusion of GABA evoked a reversible current that plateaued after 30–40 seconds. (E) No difference in maximum current density of GABA-evoked current was observed following 9–21 days of exposure to 50 mM alcohol in neurons derived from 4 control or 5 alcoholic iPSC lines. (F and G) No significant difference was observed in membrane capacitance (an indicator of cell size) or resting membrane potential in non-alcohol treated neurons derived from the 6 control and 7 alcoholic iPSC lines. (Numbers within bars indicate the total patched neurons).
To examine the effects of chronic alcohol exposure on maximum amplitude of GABA-mediated current, responses were evoked by bath application of 10 μM GABA in mature (22–36 week old) neurons from 4 CTL lines and 5 AD lines treated with either neural differentiation media or media containing 50 mM alcohol for 9–21 days, with media replaced daily. Bath application of GABA produced a robust, reversible current that plateaued approximately 30–40 sec following application (Figure 2D). There was no significant effect of prolonged alcohol exposure on maximal current density of GABA-evoked responses (p = 0.42), and there was no significant interaction between alcohol treatment and donor status (p = 0.89) (Figure 2E). Importantly, comparison of non-alcohol exposed cells derived from CTLs and ADs used for the electrophysiological experiments revealed no difference in membrane capacitance (an indicator of cell size) (p = 0.22) or resting membrane potential (p = 0.91) (Figure 2F–G), suggesting that the cells used for electrophysiological analysis were of similar developmental maturity.
Discussion
A variety of factors influence the risk for alcohol use disorder, which is heterogeneous and polygenic (Gelernter and Kranzler 2009). Alcohol research may benefit from in vitro human neural cell model systems that capture an individual’s genetic complexity to elucidate alcohol’s actions on neural cells and examine how these actions may differ in at-risk individuals. Here, we utilized iPSCs derived neural cultures from non-alcoholic CTLs and individuals diagnosed with alcohol dependence. Using an established neural differentiation protocol (Zeng et al. 2010), we examined GABAA receptor gene expression and electrophysiological function following alcohol exposure in differentiated human neural cultures enriched for forebrain-type neurons.
We found that mRNA expression of GABRA1, GABRG2, and GABRD was upregulated in iPSC-derived neural cultures following 21 days of exposure to 50 mM alcohol. Findings from previous studies using rodent in vitro and in vivo models to examine GABAA subunit expression following alcohol exposure have yielded variable results (for a detailed review, see (Grobin et al. 1998, Kumar et al. 2009). Variability in results may reflect differences in cell-type specific or brain-region specific regulation of gene expression in response to chronic alcohol exposure (Kumar et al. 2009). This work suggests that our results may only reflect transcriptional response in embryonic forebrain glutamatergic neurons. When considering inhibitory interneurons, a possibility arises that we did not observe robust changes in gene expression or electrophysiologic effects in response to prolonged alcohol exposure due to our cultures being enriched for excitatory glutamate neurons. While our electrophysiological recordings clearly demonstrate the presence of functional GABAA receptors, it may be that robust endogenous GABAergic synaptic transmission is needed during the alcohol exposure period to observe compensatory changes in expression and function. Utilizing a differentiation protocol to produce a heterogeneous population of glutamatergic and GABAergic neurons, or co-culturing enriched cultures, may provide a model system more relevant to the human brain.
Downregulation of the GABRA1 gene following alcohol exposure has often been observed in adult rodent models (Mhatre and Ticku 1992, Devaud et al. 1995, Kumar et al. 2009). In contrast, we found increased expression of GABRA1 following 21-day alcohol exposure in our human neural cell model. This difference in findings may be related to the difference in age and maturity of our neural cultures compared with adult rodent cells. Comparisons between iPSC-derived neural cells and human brain showed that the transcriptome profile of iPSC-derived neural cultures most closely resembles that of first trimester fetal brain tissue (Brennand et al. 2015), suggesting that iPSC-derived neural cultures may be a particularly suitable model to examine the toxic effects of prenatal alcohol consumption related to fetal alcohol spectrum disorder. Rodent models of fetal alcohol syndrome have reported excessive activation of GABA synaptic transmission (Ikonomidou et al. 2000) and regulation of GABAA receptor subunits including a downregulation of α5 expression in brain (Toso et al. 2006), upregulation of the β2/3 subunit in the hippocampus (Iqbal et al. 2004) and upregulation of δ expression and function in cerebellar granule neurons (Diaz et al. 2014), the latter of which was recapitulated in the current study, suggesting that iPSC-models of alcohol exposure early in development could elucidate novel effects of alcohol on relatively immature human neurons.
We observed a modestly significant interaction between alcohol treatment and iPSC donor status for GABRD mRNA with a 24% increase in RNA for AD subjects but no change for CTLs. Prior reports have demonstrated an increase in GABRD expression in the periaqueductal gray matter and dorsal raphe nucleus of alcohol preferring rats following alcohol exposure (McClintick et al. 2015, McClintick et al. 2016), while studies using non-preferring rats found no change in expression (Devaud et al. 1995, Petrie et al. 2001). Expression of GABRD mRNA was lower in the orbitofrontal cortex and caudate nucleus, but not in the hippocampus, amygdala, or putamen of post mortem brain samples from ADs compared to CTLs (Jin et al. 2012, Bhandage et al. 2014, Jin et al. 2014). This prior work, taken together with our finding that 21-day alcohol exposure increased levels of GABRD mRNA in AD neural cell lines, suggests that the regulation of the δ subunit may be relevant to the pathophysiology of alcohol use disorder. Because iPSCs reflect the donor subject’s genetic background, we may have seen an increase in GABRD in AD lines due to AD-derived neural cultures harboring a greater number of risk-associated genetic variants related to the regulation of GABRD expression than CTLs. Further studies are needed to elucidate the underlying genetic contributions of changes in GABRD regulation following alcohol exposure and to identify mechanisms linking the expression of GABRD to electrophysiological and behavioral outcomes. In this regard, it is noteworthy that GABRD has been implicated as an alcohol use disorder candidate gene (Rodd et al. 2007) and GABRD knockout mice show alcohol-related behavioral phenotypes, including reduced voluntary alcohol consumption and alcohol preference (Mihalek et al. 2001). Furthermore, pharmacological modulation of δ-containing GABAA receptors induced glutamate receptor plasticity on dopamine neurons in the ventral tegmental area suggesting that modulation of δ-containing GABAA receptors may be sufficient to induce the synaptic plasticity in this region that is commonly observed following administration of drugs of abuse (Vashchinkina et al. 2012, Vashchinkina et al. 2014).
It is possible that we did not observe robust effects of alcohol exposure on either GABAA subunit gene expression or GABA-evoked currents due to deficits in neuroactive steroid signaling in iPSC-derived neural cultures. Exposure to alcohol stimulates the production of GABAA-receptor modulating neurosteroids (Morrow et al. 2001, Sanna et al. 2004, Morrow et al. 2006, Tokuda et al. 2011), which, in part, mediate the potentiating effects of alcohol at GABAA receptors in rodent brain slice preparations (Sanna et al. 2004). Prolonged exposure to neurosteroids produces GABAA subunit gene expression changes that mimic alcohol exposure, and can be blocked by the inhibition of neurosteroid biosynthesis with finasteride (Yu et al. 1996, Follesa et al. 2000, Follesa et al. 2004), suggesting that some of the effects of alcohol on GABAA receptor function and gene expression may be mediated through neurosteroids. Further, using brief applications of the synthetic neuroactive steroids gaboxadol and ganaxolone together with GABRD knock out mice Vashchinkina et al., (2012 and 2014) have shown that neurosteroid activation of GABRD receptors results in long-lasting glutamate receptor synaptic plasticity in ventral tegmental area dopamine neurons, including changes in AMPA/NMDA glutamate signaling ratios similar to those induced by acute alcohol exposure, further implicating endogenous neuroactive steroids in the neuroplastic effects of alcohol. Therefore, we may not have observed a robust effect of alcohol on GABAA receptor expression or function because our iPSC neural culture system may lack endogenous neurosteroids or neurosteroid precursors necessary to fully manifest alcohol’s effects. Future work could address this by examining changes in GABAA receptor gene expression following chronic treatment with neurosteroids or by examining the effect of alcohol in cultures containing neuronal media supplemented with upstream precursors for neurosteroid synthesis.
Strengths of the current study include its novel examination of acute and chronic effects of alcohol on the GABAA receptor in human neural cells in vitro and the examination of iPSC-derived neural cell lines from a cohort of 11 control and 13 alcoholic donor subjects. Our findings should be considered in light of several limitations, including that GABA-mediated currents were evoked via puff application of GABA with the micropipette targeted at the cell soma, which allowed us to examine only the postsynaptic effects of alcohol on primarily somatic GABAA receptor function. Presynaptic actions of alcohol have been reported, including increased the frequency of spontaneous inhibitory events (Sanna et al. 2004, Zhu and Lovinger 2006, Criswell et al. 2008) and increasing the amount of GABA released from presynaptic terminals (Nie et al. 2004). The presynaptic effects of alcohol may be mediated though actions on metabotropic glutamate and GABAB receptors (Nie et al. 2000, Zhu and Lovinger 2006). By exogenously applying GABA to evoke a current, we could examine only postsynaptic GABAA receptor function. Future studies using iPSC-derived neurons could extend our findings by examining the effects of alcohol on endogenous inhibitory synaptic transmission, including the frequency and amplitude of spontaneous inhibitory currents. Using drugs targeting presynaptic metabotropic GABAB receptors in conjunction with alcohol could aid in differentiating presynaptic from postsynaptic effects of alcohol in this model system. Although our method of puff-application of GABA to evoke a postsynaptic current targeted primarily somatic GABAA receptors, rodent models suggest that alcohol effects are greater on the potentiation of GABAA receptors located on the soma and proximal vs. distal dendrites (Weiner et al. 1997, Wu et al. 2005). Although these finding suggest that somatic and proximal GABAA receptors are more sensitive to the effects of alcohol, it remains to be determined whether these findings translate into human iPSC-derived neurons. Future studies could examine the possible sub-localization of more alcohol-sensitive GABAA receptors by focal puff application of GABA onto different regions of the neuron.
An additional limitation was that we examined only three genes encoding GABAA receptor subunits (GABRA1, GABRG2, and GABRD). These genes were selected because their expression is reliably detected in our iPSC-derived neural culture system and because prior studies reported differences in their expression following alcohol exposure (for review, see (Kumar et al. 2009). We did not examine the effects of alcohol on the expression of GABAA subunit-encoding genes located on chromosome 4 due to our prior observation of large basal expression differences of genes within this cluster among iPSC-derived neural cell lines (Lieberman et al. 2015). The variability in expression of chromosome 4 GABA genes among iPSC lines (Lieberman et al. 2015), with very low to undetectable levels in 40% of lines, was an unforeseen limitation of the model system that prevented us from examining these genes of interest in the current study.
Furthermore, based on our current experimental methods it is not possible to easily relate changes observed in gene expression following alcohol to functional adaptations that may relate to the development of tolerance. Due to the extensive recording time required for whole cell patch clamp electrophysiology we needed to examine cells over a range of alcohol exposure lengths (9–21 days) in order to accumulate sufficient number of cell recordings for each neural cell line. Cells used for electrophysiological analysis were also maintained longer in culture prior to alcohol exposure to enhance the maturation of neuronal properties (Fink et al. 2017). Whether molecular adaptations in control and alcoholic-derived neurons lead to functional electrophysiological differences might be better examined by use of GABAA subunit-selective agonists and antagonists during electrophysiology recordings following chronic alcohol exposure, such as the GABAA δ-subunit-preferring agonist gaboxadol (THIP) (Chandra et al. 2010, Diaz et al. 2014).
Because iPSC culture is costly and time consuming, we were limited to the examination of a single concentration of alcohol (50 mM), which we selected because it has been used for in vitro studies using rodent neurons examining the effects of alcohol on GABA receptor expression and function, and because we used this concentration in our previous study examining alcohol’s actions on NMDA receptors in iPSC-derived neural cell lines (Lieberman et al. 2012). The 50 mM concentration corresponds to a breath alcohol concentration of 0.24 g/dL, which is three times the legal driving limit of 0.08 in most U.S. states. Lower concentrations of alcohol (<10 mM) that are reached with social drinking have been shown to target extrasynaptic δ subunit-containing GABAA receptors (Sundstrom-Poromaa et al. 2002, Wei et al. 2004). Future studies could utilize iPSC-derived neural cells to examine the effects of low concentrations of alcohol, which may be more relevant to the initiation of drinking, and may help to elucidate cellular mechanisms important in the transition from social drinking to the development of an alcohol use disorder.
In summary, we utilized human iPSC-derived neural cell lines from CTL and AD donor subjects and found that chronic treatment with 50 mM alcohol increased the expression of α1 (GABRA1) and γ2 (GABRG2) subunit mRNA in both groups of neural cell lines, while expression of δ (GABRD) subunit mRNA was increased only in AD cell lines. Acute and chronic alcohol exposure had no effect on GABA-evoked currents examined via whole cell patch-clamp electrophysiology. This work supports the application of iPSCs to the study of alcohol use disorder, but suggests that future work should employ additional neural differentiation protocols that may generate more alcohol-responsive neural cell types to better elucidate molecular phenotypes that differentiate CTL- and AD-derived cell cultures. Additional evaluation of neural media supplements, including neurosteroid precursors, may help to improve the utility of in vitro cultures in recapitulating the in vivo milieu.
Highlights.
iPSCs were generated from control and alcoholic donor subjects
iPSCs differentiate into neural cells enriched for functional glutamate neurons
GABAA subunit gene expression was increased following 21 day alcohol exposure
No effect of acute or chronic alcohol exposure was observed on GABA-evoked currents
Acknowledgments
We would like to thank Leann Crandall at the UC Stem Cell Core for her valued assistance in generating iPSC lines. Supported by NIH grants P60 AA03510 (Alcohol Research Center), AA23192 (HRK), AA015606 (JC), M01 RR06192 (University of Connecticut General Clinical Research Center) and by a grant from the CT Department of Public Health (JC).
Footnotes
conflicts of interest
Dr. Kranzler has served as a consultant, CME speaker, or advisory board member for the following companies: Indivior, Lundbeck, and Pfizer. He is a member of the Alcohol Clinical Trials Group of the American Society of Clinical Psychopharmacology, which is supported by Abbvie, Alkermes, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, and Xenoport.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguayo LG. Ethanol potentiates the GABAA-activated Cl- current in mouse hippocampal and cortical neurons. Eur J Pharmacol. 1990;187(1):127–130. doi: 10.1016/0014-2999(90)90349-b. [DOI] [PubMed] [Google Scholar]
- Bhandage AK, Jin Z, Bazov I, Kononenko O, Bakalkin G, Korpi ER, Birnir B. GABA-A and NMDA receptor subunit mRNA expression is altered in the caudate but not the putamen of the postmortem brains of alcoholics. Front Cell Neurosci. 2014;8:415. doi: 10.3389/fncel.2014.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, Beaumont KG, Kim HJ, Topol A, Ladran I, Abdelrahim M, Matikainen-Ankney B, Chao SH, Mrksich M, Rakic P, Fang G, Zhang B, Yates JR, 3rd, Gage FH. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. 2015;20(3):361–368. doi: 10.1038/mp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63(1):53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Chandra D, Halonen LM, Linden AM, Procaccini C, Hellsten K, Homanics GE, Korpi ER. Prototypic GABA(A) receptor agonist muscimol acts preferentially through forebrain high-affinity binding sites. Neuropsychopharmacology. 2010;35(4):999–1007. doi: 10.1038/npp.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Pond T, Feinn R, Arias AJ, Oncken C, Kranzler HR. Dutasteride reduces alcohol’s sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology (Berl) 2014;231(17):3609–3618. doi: 10.1007/s00213-014-3487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Griffith BL, Breese GR. Comparison of effect of ethanol on N-methyl-D-aspartate- and GABA-gated currents from acutely dissociated neurons: absence of regional differences in sensitivity to ethanol. J Pharmacol Exp Ther. 2003;304(1):192–199. doi: 10.1124/jpet.102.041590. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Kelm MK, Breese GR. Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. J Pharmacol Exp Ther. 2008;326(2):596–603. doi: 10.1124/jpet.107.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of gamma-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol Pharmacol. 1995;48(5):861–868. [PubMed] [Google Scholar]
- Diaz MR, Vollmer CC, Zamudio-Bulcock PA, Vollmer W, Blomquist SL, Morton RA, Everett JC, Zurek AA, Yu J, Orser BA, Valenzuela CF. Repeated intermittent alcohol exposure during the third trimester-equivalent increases expression of the GABA(A) receptor delta subunit in cerebellar granule neurons and delays motor development in rats. Neuropharmacology. 2014;79:262–274. doi: 10.1016/j.neuropharm.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90(1):95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Zhou Z, Kimura M, Mash DC, Yuan Q, Goldman D. GABAergic gene expression in postmortem hippocampus from alcoholics and cocaine addicts; corresponding findings in alcohol-naive P and NP rats. PLoS One. 2012;7(1):e29369. doi: 10.1371/journal.pone.0029369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink JJ, Robinson TM, Germain ND, Sirois CL, Bolduc KA, Ward AJ, Rigo F, Chamberlain SJ, Levine ES. Disrupted neuronal maturation in Angelman syndrome-derived induced pluripotent stem cells. Nat Commun. 2017;8:15038. doi: 10.1038/ncomms15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Caria S, Gorini G, Biggio G. Modulation of GABA(A) receptor gene expression by allopregnanolone and ethanol. Eur J Pharmacol. 2004;500(1–3):413–425. doi: 10.1016/j.ejphar.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol. 2000;57(6):1262–1270. [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR. Genetics of alcohol dependence. Hum Genet. 2009;126(1):91–99. doi: 10.1007/s00439-009-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139(1–2):2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanolinduced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287(5455):1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Iqbal U, Dringenberg HC, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure alters hippocampal GABA(A) receptors and impairs spatial learning in the guinea pig. Behav Brain Res. 2004;150(1–2):117–125. doi: 10.1016/S0166-4328(03)00246-8. [DOI] [PubMed] [Google Scholar]
- Jin Z, Bazov I, Kononenko O, Korpi E, Bakalkin G, Birnir B. Selective Changes of GABAA Channel Subunit mRNAs in the Hippocampus and Orbitofrontal Cortex but not in Prefrontal Cortex of Human Alcoholics. Frontiers in Cellular Neuroscience. 2012;5(30):30. doi: 10.3389/fncel.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Bhandage AK, Bazov I, Kononenko O, Bakalkin G, Korpi ER, Birnir B. Expression of specific ionotropic glutamate and GABA-A receptor subunits is decreased in central amygdala of alcoholics. Front Cell Neurosci. 2014;8:288. doi: 10.3389/fncel.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205(4):529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Levine ES. BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. J Neurophysiol. 2010;104(4):1923–1932. doi: 10.1152/jn.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R, Kranzler HR, Joshi P, Shin DG, Covault J. GABRA2 Alcohol Dependence Risk Allele is Associated with Reduced Expression of Chromosome 4p12 GABAA Subunit Genes in Human Neural Cultures. Alcohol Clin Exp Res. 2015;39(9):1654–1664. doi: 10.1111/acer.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R, Levine ES, Kranzler HR, Abreu C, Covault J. Pilot Study of iPS-Derived Neural Cells to Examine Biologic Effects of Alcohol on Human Neurons In Vitro. Alcohol Clin Exp Res. 2012;36(10):1678–1687. doi: 10.1111/j.1530-0277.2012.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, McBride WJ, Bell RL, Ding ZM, Liu Y, Xuei X, Edenberg HJ. Gene expression changes in serotonin, GABA-A receptors, neuropeptides and ion channels in the dorsal raphe nucleus of adolescent alcohol-preferring (P) rats following binge-like alcohol drinking. Pharmacol Biochem Behav. 2015;129:87–96. doi: 10.1016/j.pbb.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, McBride WJ, Bell RL, Ding ZM, Liu Y, Xuei X, Edenberg HJ. Gene Expression Changes in Glutamate and GABA-A Receptors, Neuropeptides, Ion Channels, and Cholesterol Synthesis in the Periaqueductal Gray Following Binge-Like Alcohol Drinking by Adolescent Alcohol-Preferring (P) Rats. Alcohol Clin Exp Res. 2016;40(5):955–968. doi: 10.1111/acer.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK. Chronic ethanol administration alters gamma-aminobutyric acidA receptor gene expression. Mol Pharmacol. 1992;42(3):415–422. [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK. Chronic GABA treatment downregulates the GABAA receptor alpha 2 and alpha 3 subunit mRNAS as well as polypeptide expression in primary cultured cerebral cortical neurons. Brain Res Mol Brain Res. 1994;24(1–4):159–165. doi: 10.1016/0169-328x(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, Homanics GE. GABA(A)-receptor delta subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25(12):1708–1718. [PubMed] [Google Scholar]
- Milivojevic V, Feinn R, Kranzler HR, Covault J. Variation in AKR1C3, which encodes the neuroactive steroid synthetic enzyme 3alpha-HSD type 2 (17beta-HSD type 5), moderates the subjective effects of alcohol. Psychopharmacology (Berl) 2014;231(17):3597–3608. doi: 10.1007/s00213-014-3614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8(4):463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37(1–3):98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol enhances gamma-aminobutyric acid responses in a subpopulation of nucleus accumbens neurons: role of metabotropic glutamate receptors. J Pharmacol Exp Ther. 2000;293(2):654–661. [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303(5663):1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56(1):141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oni EN, Halikere A, Li G, Toro-Ramos AJ, Swerdel MR, Verpeut JL, Moore JC, Bello NT, Bierut LJ, Goate A, Tischfield JA, Pang ZP, Hart RP. Increased nicotine response in iPSC-derived human neurons carrying the CHRNA5 N398 allele. Sci Rep. 2016;6:34341. doi: 10.1038/srep34341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie J, Sapp DW, Tyndale RF, Park MK, Fanselow M, Olsen RW. Altered gabaa receptor subunit and splice variant expression in rats treated with chronic intermittent ethanol. Alcohol Clin Exp Res. 2001;25(6):819–828. [PubMed] [Google Scholar]
- Picton AJ, Fisher JL. Effect of the alpha subunit subtype on the macroscopic kinetic properties of recombinant GABA(A) receptors. Brain Res. 2007;1165:40–49. doi: 10.1016/j.brainres.2007.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bertsch BA, Strother WN, Le-Niculescu H, Balaraman Y, Hayden E, Jerome RE, Lumeng L, Nurnberger JI, Jr, Edenberg HJ, McBride WJ, Niculescu AB. Candidate genes, pathways and mechanisms for alcoholism: an expanded convergent functional genomics approach. Pharmacogenomics J. 2007;7(4):222–256. doi: 10.1038/sj.tpj.6500420. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24(29):6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Filichia E, Shick E, Preston KL, Phillips KA, Cooperman L, Lin Z, Tesar P, Hoffer B, Luo Y. Using iPSC-derived human DA neurons from opioid-dependent subjects to study dopamine dynamics. Brain Behav. 2016;6(8):e00491. doi: 10.1002/brb3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5(8):721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J Neurosci. 2011;31(27):9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso L, Roberson R, Woodard J, Abebe D, Spong CY. Prenatal alcohol exposure alters GABA(A)alpha5 expression: a mechanism of alcohol-induced learning dysfunction. Am J Obstet Gynecol. 2006;195(2):522–527. doi: 10.1016/j.ajog.2006.01.098. [DOI] [PubMed] [Google Scholar]
- Vashchinkina E, Manner AK, Vekovischeva O, den Hollander B, Uusi-Oukari M, Aitta-Aho T, Korpi ER. Neurosteroid Agonist at GABAA receptor induces persistent neuroplasticity in VTA dopamine neurons. Neuropsychopharmacology. 2014;39(3):727–737. doi: 10.1038/npp.2013.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashchinkina E, Panhelainen A, Vekovischeva OY, Aitta-aho T, Ebert B, Ator NA, Korpi ER. GABA site agonist gaboxadol induces addiction-predicting persistent changes in ventral tegmental area dopamine neurons but is not rewarding in mice or baboons. J Neurosci. 2012;32(15):5310–5320. doi: 10.1523/JNEUROSCI.4697-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low-dose alcohol actions on alpha4beta3delta GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci U S A. 2006;103(22):8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24(38):8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1997;77(3):1306–1312. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111(3):533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Zhang L, Carlen PL. Potentiation of GABAA-mediated synaptic current by ethanol in hippocampal CA1 neurons: possible role of protein kinase C. J Pharmacol Exp Ther. 1994;268(3):1388–1395. [PubMed] [Google Scholar]
- Wu PH, Poelchen W, Proctor WR. Differential GABAB Receptor Modulation of Ethanol Effects on GABA(A) synaptic activity in hippocampal CA1 neurons. J Pharmacol Exp Ther. 2005;312(3):1082–1089. doi: 10.1124/jpet.104.075663. [DOI] [PubMed] [Google Scholar]
- Xie Z, Li G, Ye J-H. Acute effects of ethanol on GABAA and glycine currents in the lateral habenula neurons of young rats. Open Journal of Neuroscience. 2013;3(1) doi: 10.13055/ojns_3_1_5.130821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HH, Kolb JE. Ethanol modulation of GABA-activated current responses in acutely dissociated retinal bipolar cells and ganglion cells. Alcohol Clin Exp Res. 1997;21(4):647–655. [PubMed] [Google Scholar]
- Yu R, Follesa P, Ticku MK. Down-regulation of the GABA receptor subunits mRNA levels in mammalian cultured cortical neurons following chronic neurosteroid treatment. Brain Res Mol Brain Res. 1996;41(1–2):163–168. doi: 10.1016/0169-328x(96)00087-3. [DOI] [PubMed] [Google Scholar]
- Zeng H, Guo M, Martins-Taylor K, Wang X, Zhang Z, Park JW, Zhan S, Kronenberg MS, Lichtler A, Liu HX, Chen FP, Yue L, Li XJ, Xu RH. Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells. PLoS ONE. 2010;5(7):e11853. doi: 10.1371/journal.pone.0011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96(1):433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]