Abstract
In murine model systems, inducible costimulator (ICOS) signaling has been implicated in the formation of chronic graft-versus-host disease (GVHD). Previously, we showed that chronic GVHD can be reproducibly produced in the dog hematopoietic cell transplantation (HCT) model and that ICOS expression is upregulated on T cells in dogs with chronic GVHD. The goal of the present study was to determine whether administration of a short course of anti-canine ICOS monoclonal antibody (mAb) could alter the rapid and progressive course of chronic GVHD. Five dogs underwent HCT from dog leukocyte antigen mismatched unrelated donors following total body irradiation. Post-grafting immunosuppression consisted of methotrexate (days 1, 3, 6, and 11) and cyclosporine (days -1 through 78). Anti-ICOS mAb (3 injections, 72 hours apart) was administered upon diagnosis of GVHD. One dog failed to respond to anti-ICOS mAb therapy and succumbed to chronic GVHD in a time course similar to control untreated dogs. Overall, anti-ICOS-treated dogs experienced a significant prolongation in survival from the time of diagnosis of chronic GVHD compared to control dogs. Within the limitations of the number of study dogs, we suggest that a short course of anti-ICOS mAb may be useful in the treatment of chronic canine GVHD.
Keywords: GVHD, anti-ICOS, canine, HCT
INTRODUCTION
We recently described a canine model of chronic graft-versus-host disease (GVHD) after hematopoietic cell transplantation (HCT) from unrelated donors that were mismatched for the major histocompatibility complex (MHC), dog leukocyte antigen (DLA) [1]. Recipients were conditioned for transplantation with 9.2 Gy total body irradiation (TBI) and were given post-grafting immunosuppression with a short course of methotrexate (MTX) and 80 days of cyclosporine (CSP). Eight of 9 dogs developed chronic GVHD and, given both the MHC disparity and the absence of specific treatment, succumbed to chronic GVHD a median of 10 days after diagnosis. In an earlier publication [2], we reported up-regulation of inducible costimulator (ICOS) on activated T cells in dogs with chronic GVHD. Here, we asked whether the natural history of chronic GVHD in this model could be altered and survival extended by a short course of treatment with an anti-ICOS monoclonal antibody (mAb).
MATERIALS AND METHODS
Experimental Animals
Random-bred litters of beagles and mini-mongrel cross-breeds were raised at the Fred Hutchinson Cancer Research Center, Seattle WA. The dogs weighed from 6.7 to 8.4 (median, 7.9) kg and were 10.4 to 20.3 (median, 17.4) months old. They were observed for disease at least 20 days before study. The Institutional Care and Use Committee of the Fred Hutchinson Cancer Research Center approved the research protocols and the American Association for the Accreditation of Laboratory Animal Care certified the facility. Five donors and five recipients were unrelated for at least five generations and were mismatched for highly polymorphic major histocompatibility complex (dog leukocyte antigen [DLA]) class I and class II associated microsatellite markers [3,4]. DLA mismatching was confirmed by direct sequencing for DLA-DRB1 alleles [5].
DLA-Mismatched Unrelated HCT
HCT was performed identically to that previously reported [1]. Five days before and up to five days after transplantation, dogs were prophylactically treated with the antibiotic, enrofloxacin (2.2 mg/kg subcutaneously, twice daily). On day 0, HCT recipients were conditioned with a single dose of 9.2Gy total body irradiation (TBI) delivered at a rate of 7 cGy/minute from a high-energy linear accelerator (Varian Clinac 6, Palo Alto, CA). Within 4 hours after TBI, the recipients were given an intravenous (IV) infusion of 3.0 to 5.0 × 108 (median 4.7) nucleated donor marrow cells/kg. Twenty-four hours later, the recipients were given an IV infusion of 1.3 to 3.9 × 108 (median, 3.8) peripheral blood buffy coat cells/kg obtained by COBE apheresis from the marrow donor. Postgrafting immunosuppression consisted of IV methotrexate (MTX) 0.4 mg/kg/day on days 1, 3, 6, and 11 and cyclosporine (CSP) given twice daily starting on day -1 through 78 at a dose of 7.5–15 mg/kg adjusted to maintain a blood CSP level between 100 to 300 ng/ml. Marrow recipients were given ursodiol (0.75 mg/kg, twice daily, days -1 to 80) to mitigate liver GVHD (Figure 1). All dogs were given standard postgrafting care including constant rate infusion of lactated Ringers solution while receiving MTX. Five days after transplantation dogs were switched to prophylactic ceftazidime (37.5 mg/kg iv) and gentamicin (6 mg/kg IV) twice daily. Fevers were treated as sepsis and dogs were given antibiotics. Hematopoietic engraftment was assessed by chimerism studies using microsatellite markers [6,7].
Figure 1. Schema for induction and treatment of chronic GVHD in DLA-mismatched unrelated HCT dogs.
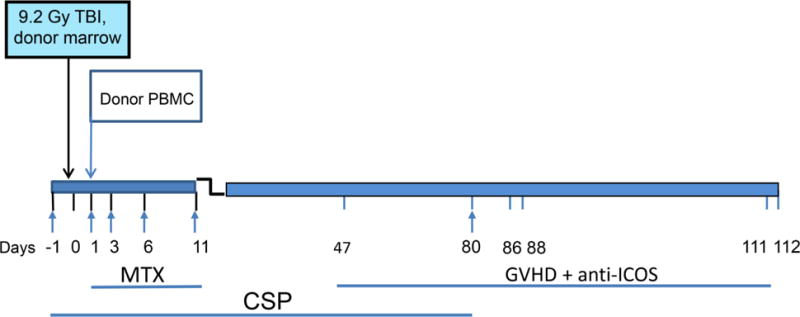
On day 0, recipients received 9.2Gy TBI and donor marrow. On day 1 recipient dogs were given apheresed donor buffy coat cells obtained from their respective donors. Immunosuppression (arrows) consisted of 0.4 mg/kg MTX injected IV on days 1, 3, 6, 11, and oral CSP given on days -1 to 78 at dosing designed to maintain serum levels at 100–300 ng/ml. Oral ursodiol was given on days -1 to 80 to reduce the incidence of liver GVHD. Clinical signs of GVHD occurred from days 47 to 112 (tick marks).
Evaluation of Chronic GVHD and Anti-ICOS mAb Therapy
A diagnosis of chronic GVHD was based on clinical findings which included generalized skin ulcerations, facial edema, dry eye syndrome, erythema of the sclera, rhinitis, gingivitis, elevated liver enzymes, anorexia, vomiting and or diarrhea. The dogs were monitored at a minimum twice daily and the progression of chronic GVHD was recorded in a digital program DVMax (Veterinary Health Management Software, Westbrook, ME). Once chronic GVHD was established clinically, dogs were administered 3 injections IV of 4.0 mg/kg anti-ICOS mAb, each injection separated by 72 hours (Figure 1). When chronic GVHD of the skin was apparent, confirmation was established by skin biopsy and evaluation by histopathology. Dogs were monitored for remission or progression of disease at a minimum of twice daily. A consensus decision to euthanize an HCT recipient was made by the principle investigator, clinical veterinarian, and animal technicians currently on hand when diminished activity, failure to eat, a greater than 30% loss of weight, or signs of distress were observed. Following euthanasia, a complete necropsy was performed and representative tissues fixed in 10% buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin for evaluation by two pathologists (G.S., S.P.). Chronic GVHD was graded from mild to severe based on the degree of lymphocyte infiltration and the degree of tissue damage (apoptosis, necrosis, fibrosis, granulation tissue formation, lichenoid and sclerodermatous lesions).
ICOS Pharmacokinetics and Dog Antibody Response
The blood pharmacokinetics of anti-ICOS and the presence of dog anti-mouse antibodies (DAMA) to administered anti-ICOS were measured by standard ELISA methods [8]. For pharmacokinetics, sera were incubated on goat anti-mouse kappa antibody coated plates. Anti-ICOS mAb was detected using horseradish peroxidase-labeled goat anti-mouse IgG2a antibody, and serum levels were calculated using a standard curve generated from the anti-ICOS mAb. For DAMA titers, serial dilutions of serum were incubated on mouse anti-dog antibody-coated plates and dog IgG was detected using horseradish peroxidase-labeled goat anti-dog IgG antibody. DAMA titers were determined by comparison to untreated normal dog serum and reported as a fold change above normal responses.
RESULTS
Hematopoietic Cell Engraftment and Donor Cell Chimerism
All five dogs showed sustained hematopoietic cell engraftment after HCT. Mononuclear and granulocyte recovery for the five study dogs occurred within the same period and tempo as was seen in a group of ten transplanted dogs that were not given ICOS mAb therapy following development of chronic GVHD [1]. Donor cell chimerism values, 94 to 100 (median 100) percent, were comparable to that of the control dogs as previously reported (data not shown) [1].
Chronic GVHD and Anti-ICOS Therapy
Clinical course
Table 1 summarizes the outcomes of five dogs that developed chronic GVHD and received anti-ICOS mAb therapy. The time to development of chronic GVHD ranged from 47 to 111 (mean 89) days.
Table 1.
Effect of Anti-ICOS on GVHD
| Diagnosis of chronic GVHD | Recurrence of GVHD | Chronic GVHD | |||||
|---|---|---|---|---|---|---|---|
| Dog | Day† | Organ | Response to 1st anti-ICOS | Day† | Clinical Pathol | Day Euthanized | Organ Involvement |
| Anti-ICOS-treated | |||||||
| H789 | 86* | SK, SC | No | NA | N.A. | 98 | SK, LV, LU, ES,PA,CN |
| H799 | 88* | SK,SC | complete | 193 * | SK, SC | 212 | SK, SC, ES, LU, GI, HR |
| H800 | 47* | GI | complete | 87 * | SK, AP | 136 | SK, SG, LU, LV, ES, LG |
| H803 | 112* | SK, SC | complete | 148 | SK | 175 | SK, ES, GI |
| H805 | 111* | GV | complete | 125 | SK, SC | 150 | SK, ES, LV, SG, LG |
| Controls‡ | Clinical Diagnosis | End of Study Histopathology | |||||
| H642 | 66 | SK, GV | NA | NA | NA | 74 | SK, LV, LU, ES |
| H634 | 88 | GV, CN | NA | NA | NA | 103 | SK, SG, LV |
| H678 | 81 | SK, CN | NA | NA | NA | 91 | SK, LV, LU, GI |
| H694 | NA | SK | NA | NA | NA | 106 | SK, SG, LV, LU, LG |
| H708 | 98 | SK, CN | NA | NA | NA | 101 | SK, SG, LV, LU, ES, GI |
| H720 | 43 | SK | NA | NA | NA | 55 | SK, SG, LV, LU, LG |
| H709 | 164 | SK | NA | NA | NA | 189 | SK, SG, LU, LG, ES |
| H698 | 114 | SK, CN | NA | NA | NA | 121 | SK, SG, LV, LU, ES, GI |
Abbreviations: AP = alopecia; CN = conjunctiva; ES = esophagus; GI = gastrointestinal; GV = gingiva; HR = Heart; LG = lacrimal gland; LV= liver; LU = lung; SC = sclera of eyes; SG = salivary gland; SK = Skin; PA = pancreas;
Start of anti-ICOS mAb infusion.
Days from HCT
Control group [1].
Dog H789 received a single course of three injections of anti-ICOS mAb on day 86 after HCT once chronic GVHD was apparent in the skin and sclera. There was no response, and the disease progressed. By day 98, the dog was euthanized (12 days after the initial diagnosis of chronic GVHD). Histopathology revealed chronic GVHD of skin (lymphocytic interface dermatitis and folliculitis), liver (lymphoplasmacytic triaditis), lung (bronchiolitis obliterans), pancreas (lymphocytic infiltrates with acinar loss and fibrosis) and esophagus (lichenoid lymphocytic infiltrates with adenitis, dochitis, and gland loss), and conjunctiva (lichenoid lymphocytic conjunctivitis).
Dog H799 first showed evidence of chronic GVHD on day 88 after HCT. The first course of 3 injections of anti-ICOS mAb was begun on day 91. An abdominal skin biopsy collected 4 days after the third injection of anti-ICOS revealed still-active GVHD characterized by extensive involvement of the hair follicles. A second skin biopsy collected 3 days later showed much less active signs of GVHD than the first biopsy (Figure 2). Resolution and recovery from the initial signs of skin GVHD continued but gradually returned by day 193. Chronic GVHD was clearly present in the skin concurrent with alopecia. A second course of anti-ICOS mAb was initiated; however, an ELISA assay revealed high titers of dog anti-ICOS antibodies, DAMA, and further anti-ICOS mAb treatment was aborted. Skin GVHD resolved. However, by day 212 after HCT (124 days after initial diagnosis of chronic GVHD), the dog had to be euthanized because of extensive chronic GVHD. Necropsy findings included GVHD of the esophagus (lymphoplasmacytic adenitis and ganglionitis), salivary gland (lymphoplasmcytic adenitis), skin (lymphoplasmacytic and neutrophilic interface dermatitis and folliculitis), lungs (emphysema, histiocytosis), liver (lymphoplasmatic and neutrophilic cholangiohepatitis, vacuolar degeneration), and heart (lymphoplasmacytic and histiocytic myocarditis).
Figure 2.
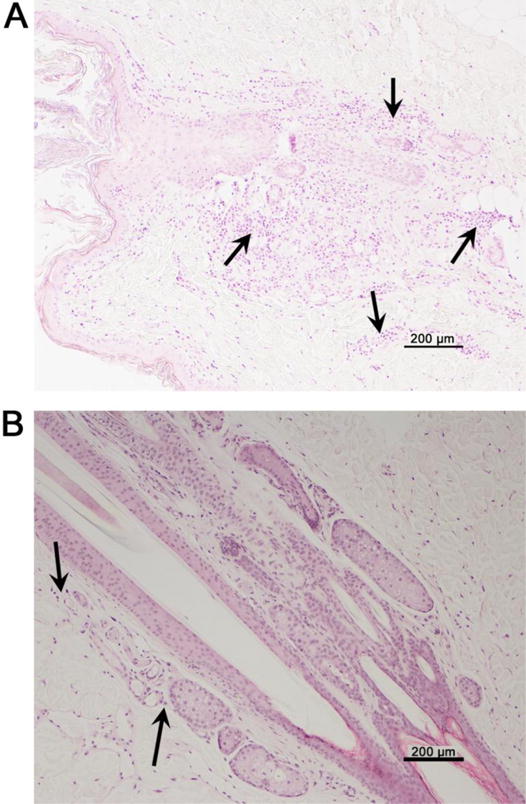
Histopathology of abdominal skin biopsies demonstrating the effect of anti-ICOS on chronic GVHD of the skin. (A) Skin biopsies from dog H799 collected four days after the third injection of anti-ICOS injection show dense lymphocytic infiltration surrounding the pilo-sebaceous structure and follicular epithelium (arrows). Lymphocytes are present in the superficial and deep dermis and exocytosis of the epidermis is multifocal. (B) The biopsy collected seven days after the third injection of anti-ICOS reveals minimal numbers of lymphocytes surrounding the pilo-sebaceous structures, few lymphocytes in the superficial dermis, and no epidermal exocytosis is present. Magnification is 100×.
Dog H800 developed symptoms of intestinal GVHD by day 47 after HCT with severe diarrhea and vomiting. The dog was given anti-ICOS mAb with continued CSP treatment until day 80. Gastrointestinal symptoms of GVHD resolved, but by day 87 clinical signs of GVHD recurred with skin lesions and alopecia. A second full course of anti-ICOS mAb was then administered. Chronic GVHD was confirmed by histopathological examination of a skin biopsy on day 90 revealing mild lymphocytic infiltration of skin and hair follicles. Progression of the disease was again halted until day 120 when chronic GVHD progressed significantly with erythema of the inside of the ears and flaky scabbed lesions of the abdomen. A second biopsy revealed progression of chronic GVHD with marked lymphocytic folliculitis and loss with mild dermal fibrosis. The dog was euthanized on day 136 (89 days after initial diagnosis of chronic GVHD) and histopathology revealed progressive chronic GVHD of the skin (lymphoplasmacytic interface dermatitis), salivary gland (lymphocytic adenitis), lung (lymphocytic and neutrophilic bronchiolitis and bronchitis), liver (lymphoplasmacytic triaditis), lacrimal glands (lymphoplasmacytic adenitis with gland loss), and esophagus (lymphoplasmacytic adenitis and dochitis with gland loss and ectasia).
H803 was diagnosed with chronic GVHD on day 112 after HCT with involvement of both the skin and the sclera. Symptoms of GVHD resolved after anti-ICOS therapy with subsequent recurrence on day 148. (63 days after initial diagnosis of chronic GVHD). A second round of anti-ICOS mAb therapy was not administered. On day 175 after HCT the dog was euthanized with extensive GVHD of the skin (lichenoid interface dermatitis), esophagus (lymphoplasmcytic infiltrates and adenitis), and gastrointestinal tract (lymphoplasmacytic ileitis and colitis).
H805 was started on anti-ICOS mAb treatment on day 111 following HCT after presenting with severe gingival GVHD with halitosis. Lesions completely responded to anti-ICOS mAb treatment within 7 days. The gingiva appeared normal and halitosis was absent. However, by day 125 scabbed lesions began to appear on the skin and gingivitis recurred. On day 151 GVHD was sufficiently advanced for euthanasia (39 days after initial diagnosis of chronic GVHD). Necropsy revealed multi-organ GVHD of the skin (lymphoplasmacytic interface dermatitis, folliculitis, sebaceous adenitis), esophagus (lymphoplasmacytic adenitis and dochitis), liver (lymphoplasmacytic triaditis), salivary gland (sialadenitis with necrosis, ganglionitis), and lacrimal gland (adenitis and dochitis).
Pharmacokinetics of anti-ICOS
Blood was collected from dogs at hourly and daily time points following each of three injections of anti-ICOS (Figure 3). The mean serum levels of anti-ICOS mAb did not change significantly over the 9-day course of injections and sampling. These results suggest that an anti-mouse antibody response failed to develop over the 9-day period and that blood levels of anti-ICOS in the 25–75 μg/ml range are effective in treating chronic GVHD.
Figure 3. Serum levels of α-ICOS.
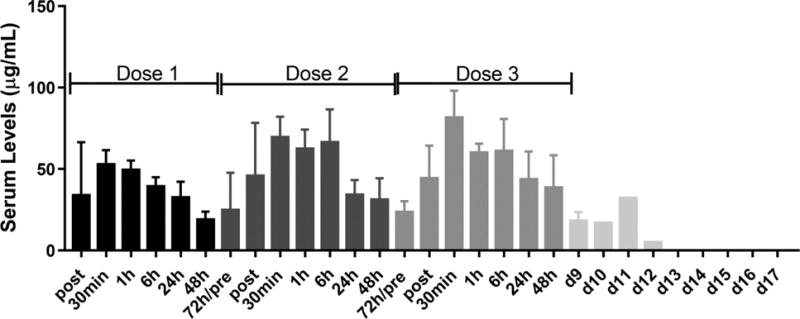
Blood was collected from dogs within 2 minutes and at 0.5, 1, 6, 24, 48, and 72 hours after injection of anti-ICOS mAb (4 mg/kg, IV) for the first 2 doses. For the third dose, the 72 hour time point was replaced by a day 9 draw. One dog provided blood at days 10 through 17 after the third injection of anti-ICOS. Serum from the blood samples was tested for levels of anti-ICOS by ELISA. Anti-ICOS levels were zero for blood samples from all 5 dogs collected before injection of ICOS. Data is presented as mean levels of antibody + standard deviation for 5 dogs.
Survival benefit of anti-ICOS
A landmark analysis compared survival of the historical untreated group of dogs with chronic GVHD [1] with the current group of dogs treated with anti-ICOS mAb at the first signs of chronic GVHD. Figure 4 shows that survival among the 8 control dogs ranged from 3 to 25 (median 10) days after diagnosis. By comparison, survival among the anti-ICOS mAb treated dogs ranged from 12 to 124 (median 30) days after diagnosis. The difference in survival of the two groups was significant at p ≤ 0.011 (Log-rank test).
Figure 4. Anti-ICOS extends survival of dogs with GVHD.
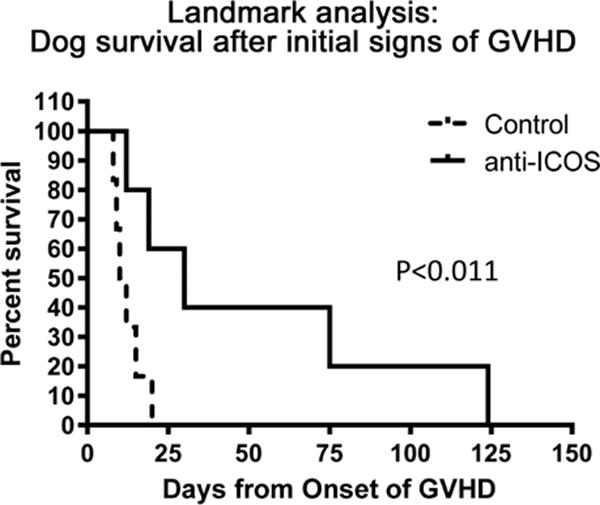
Two groups of dogs were studied from the initial diagnosis of chronic GVHD to euthanasia. In the control group, dogs, H642, H634, H678, H694, H708, H720, H709, H698, were untreated during this period aside from antibiotic and fluid support. The anti-ICOS mAb-treated group, dogs, H789, H799, H800, H803, H805, received either one or two courses of anti-ICOS mAb. The difference in the survival periods of the two groups was significant (log-rank test).
DISCUSSION
The canine HCT model used here was described recently [1] and designed to yield nearly uniform development of chronic GVHD within an economically reasonable time frame. Accordingly, transplants were from donors that were unrelated to their respective recipients for at least five generations and mismatched for the antigens of the canine MHC, DLA. Also, we shortened the time period of post-transplant CSP administration from the clinically used 180 days to 80 days in order to hasten the onset of chronic GVHD. Given the degree of MHC disparity and the shortened course of CSP immunosuppression, chronic GVHD in this model was generally rapid in onset and uniformly fatal, and effective therapy was not expected to be curative. However, as a measure of success, we expected anti-ICOS mAb therapy to temporarily reverse GVHD symptoms and extend survival.
As already indicated, previous observations in dogs with chronic GVHD had shown increased ICOS expression on T cells [2]. ICOS activation enhances all basic T cell responses including activation and proliferation, responses to foreign antigens, secretion of lymphokines, upregulation of molecules that mediate cell-cell interaction, providing effective help for antibody-secreting B-cells in response to T cell-dependent antigens, and promoting T cell homing to allografts [9–14]. Therefore, we asked whether a brief course of treatment with three injections of a murine anti-canine ICOS mAb could interrupt an ongoing GVH reaction in this model with resulting improvement in survival.
In the current small exploratory study involving five dogs with chronic GVHD, one animal did not respond at all to treatment and progressed rapidly to euthanasia within a timeframe comparable to that seen among dogs not given anti-ICOS mAb. The remaining four dogs, however, showed complete if temporary resolution of chronic GVHD manifestations with anti-ICOS mAb therapy, which recurred 14 to 80 (median 39) days later. The initial signs of remission of chronic GVHD following injection of anti-ICOS was generally rapid and occurred within a period of 4–7 days after the first injection of anti-ICOS mAb. Two closely spaced biopsies were collected from one dog, H799, after anti-ICOS therapy. The first biopsy revealed a significant lymphocytic infiltrate while the second collected 3 days later was nearly devoid of infiltrating lymphocytes. Remission of gingival chronic GVHD was also observed clinically with resolution of both gingival sores and “rotten” breath occurring within one week after injection of anti-ICOS.
Attempts at complete re-treatment were thwarted in two dogs by the development of dog anti-mouse antibodies, DAMA. Remarkably, the brief course of anti-ICOS treatment led to a significant prolongation of survival benefit compared to control dogs not given anti-ICOS mAb.
This is the first example of efficacy of anti-ICOS mAb in treating chronic GVHD in a large outbred animal model. Earlier studies in mice suggested that ICOS:ICOSL blockade could alleviate GVHD [15–17]. However, studies specifically addressing chronic GVHD were limited. Watanabi et al. [18] concluded that ICOS expression was critical for induction of chronic GVHD. This observation was based on studies using ICOS:ICOS−/− splenocytes transplanted into MHC-mismatched recipient mice, which showed a reduced ability to generate Th2-mediated chronic GVHD. Taylor et al. [19], reported that mice given 200 μg (10 mg/kg in mice versus 4 mg/kg in dogs) of anti-ICOS mAb intraperitoneally from day −1 to day +5, and then three times weekly until day 28 after marrow transplantation, resulted in a delay in mortality from GVHD. It is unknown whether the model was for acute or chronic GVHD. An in vivo investigation of the fully human anti-ICOS mAb, JTA-009, revealed that the antibody was effective in prolonging the survival of SCID mice grafted with human PBMC and reducing the level of human IFN-g in blood [20]. Our studies in the MHC-mismatched, unrelated canine HCT model further support the hypothesis that ICOS signaling plays an important role in chronic GVHD. In conclusion, the results of our study suggest that a limited course of anti-ICOS mAb is an effective inhibitor of a checkpoint thought to be important for the maintenance of chronic GVHD in the canine HCT model.
HIGHLIGHTS.
Four of five dogs with onset of chronic GVHDE responded to a short course of anti-ICOS mAb.
Two of the four dogs received a second course of anti-ICOS with additional success.
Survival from onset of chronic GVHD was significantly longer in anti-ICOS treated dogs vs controls.
Anti-ICOS may be part of an effective therapy in treating chronic GVHD.
Acknowledgments
The authors thank Alix McPhearson, Michele Spector, DVM and the Fred Hutchinson Cancer Research Center’s animal research technicians for their assistance with animal care, Stacy Zelmer and Debe Higginbotham for DLA typing and chimerism analysis, and Helen Crawford and Bonnie Larson for manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: This work was supported by grants P30 CA15704 and P01 CA78902 from the national Institutes of Health, Bethesda, MD.
Conflict Of Interest Statement: The authors have no primary financial relationships with any company that has a direct financial interest in the subject matter or with a company that produces a competing product.
References
as of 09-06-2017
- 1.Graves SS, Rezvani A, Sale G, et al. A canine model of chronic graft-vs.-host disease. Biology of Blood & Marrow Transplantation. 2016;23:420–427. doi: 10.1016/j.bbmt.2016.12.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato M, Storb R, Loretz C, et al. Inducible costimulator (ICOS) up-regulation on activated T cells in graft-versus-host disease after dog leukocyte antigen-nonidentical hematopoietic cell transplantation: a potential therapeutic target. Transplantation. 2013;96:34–41. doi: 10.1097/TP.0b013e318295c025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner JL, Burnett RC, Storb R. Molecular analysis of the DLA DR region. Tissue Antigens. 1996;48:549–553. doi: 10.1111/j.1399-0039.1996.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 4.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 5.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 6.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA) n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–706. [PubMed] [Google Scholar]
- 7.Graves SS, Hogan W, Kuhr CS, et al. Stable trichimerism after marrow grafting from 2 DLA-identical canine donors and nonmyeloablative conditioning. Blood. 2007;110:418–423. doi: 10.1182/blood-2007-02-071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorror ML, Leisenring W, Mielcarek M, et al. Intensified postgrafting immunosuppression failed to assure long-term engraftment of dog leukocyte antigen-identical canine marrow grafts after 1 gray total body irradiation. Transplantation. 2008;85:1023–1029. doi: 10.1097/TP.0b013e318169be24. [DOI] [PubMed] [Google Scholar]
- 9.Coyle AJ, Lehar S, Lloyd C, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 10.Hutloff A, Dittrich AM, Beier KC, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 11.Yoshinaga SK, Whoriskey JS, Khare SD, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 12.Coquerelle C, Oldenhove G, Acolty V, et al. Anti-CTLA-4 treatment induces IL-10-producing ICOS+ regulatory T cells displaying IDO-dependent anti-inflammatory properties in a mouse model of colitis. Gut. 2009;58:1363–1373. doi: 10.1136/gut.2008.162842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tafuri A, Shahinian A, Bladt F, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 14.Zhang QW, Rabant M, Schenk A, Valujskikh A. ICOS-Dependent and -independent functions of memory CD4 T cells in allograft rejection. American Journal of Transplantation. 2008;8:497–506. doi: 10.1111/j.1600-6143.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 15.Mollweide A, Staege MS, Hoeschen C, Hideo Y, Burdach S, Richter GH. Only therapeutic ICOS:ICOSL blockade alleviates acute graft versus host disease. Klinische Padiatrie. 2009;221:344–350. doi: 10.1055/s-0029-1239532. [DOI] [PubMed] [Google Scholar]
- 16.Fujimura J, Takeda K, Kaduka Y, et al. Contribution of B7RP-1/ICOS co-stimulation to lethal acute GVHD. Pediatric Transplantation. 2010;14:540–548. doi: 10.1111/j.1399-3046.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard VM, Eng JM, Ramirez-Montagut T, et al. Absence of inducible costimulator on alloreactive T cells reduces graft versus host disease and induces Th2 deviation. Blood. 2005;106:3285–3292. doi: 10.1182/blood-2005-01-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe S, Ogawa S, Hara Y, Tanabe K, Toma H, Abe R. Expression level of costimulatory receptor ICOS is critical for determining the polarization of helper T cell function. Transplant Immunology. 2006;15:255–263. doi: 10.1016/j.trim.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, et al. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105:3372–3380. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- 20.Tajima N, Tezuka K, Tanimoto A, et al. JTA-009, a fully human antibody against human AILIM/ICOS, ameliorates graft-vs-host reaction in SCID mice grafted with human PBMCs. Experimental Hematology. 2008;36:1514–1523. doi: 10.1016/j.exphem.2008.06.004. [DOI] [PubMed] [Google Scholar]


