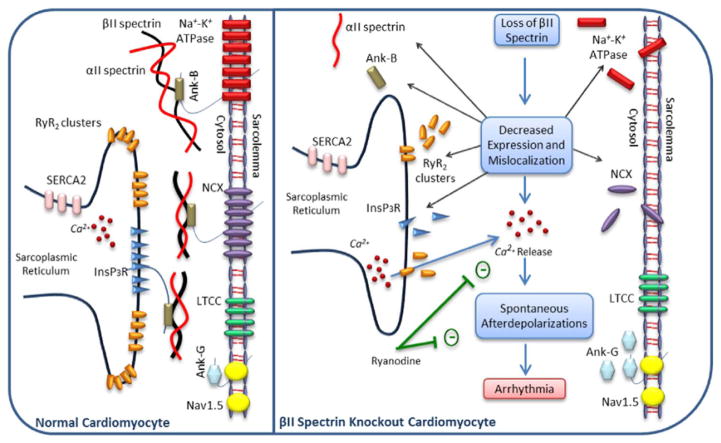
Fig. 1.
A schematic view of a normal cardiomyocyte and a βII spectrin knockout cardiomyocyte. In the absence of βII spectrin protein, the expression levels of ryanodine receptor 2 (RyR2), Na+-K+-ATPase and Na+-Ca2 +-exchanger (NCX), Ankyrin-B (Ank-B), αII spectrin are significantly decreased and showed defective localization [20]. The expression of both sarco/endoplasmic reticulum Ca2 +-ATPase (SERCA2) and voltage-gated Na+ channel (Nav1.5) are not impacted by the loss of βII spectrin [20]. Additionally, both SERCA2 and L-Type Ca2 + channel (LTCC) are properly localized. The levels of Ankyrin-G (Ank-G) are increased in the absence of βII spectrin, likely as a compensatory response to decrease ankyrin-B expression [20]. Defects in ryanodine receptors result in aberrant Ca2 +-dependent release and subsequent spontaneous afterdepolarizations which eventually lead to the development of arrhythmias. In a murine model of a cKO of βII spectrin, ryanodine was shown to inhibit Ca2+ release and abolishes these spontaneous afterdepolarizations. It is important to note that further research is warranted to demonstrate the localization of Nav1.5 and the expression of LTCC in βII spectrin deficiency. In addition, no data are currently available that directly links the expression and localization of inositol triphosphate receptor (InsP3R), a known ankyrin-B binder, to βII spectrin protein. However, mislocalization and decreased expression of InsP3R is expected due to decreased ankyrin-B expression [101,102].