Figure 5.
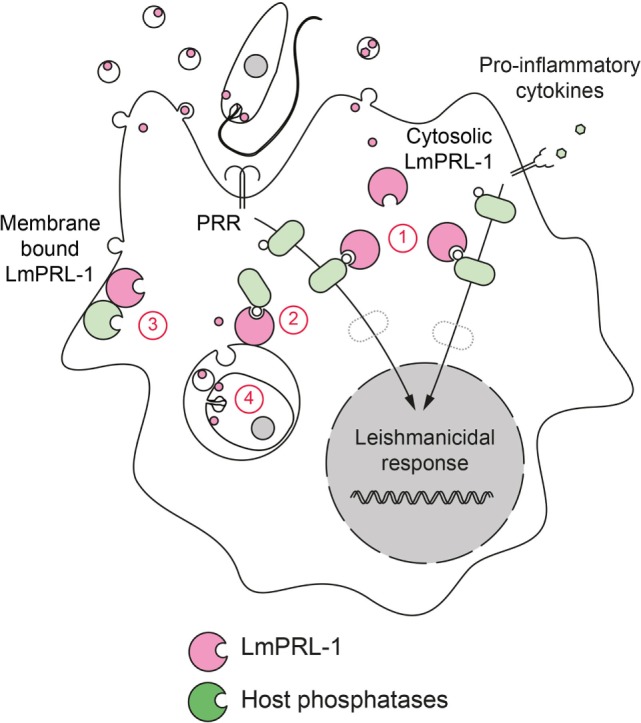
Hypotheses on the virulence mechanism of LmPRL-1. The phosphatase LmPRL-1 is secreted by Leishmania major promastigotes and amastigotes during macrophages infection. Once secreted, LmPRL-1 is localized at the membrane of the parasitophorous vacuole (PV) and/or in the cytosol of the infected macrophage. The observed support of intracellular parasite survival by LmPRL-1 could result from an inhibitory effect on important signaling pathways that otherwise promote the microbicidal response of the host macrophage (1). Other pathways not directly related to the innate immune response, but contributing to the general fitness of the macrophage and its function as host cell niche for the parasite could also be targeted by LmPRL-1 (2). Putative targets of LmPRL-1 are the host PRL proteins. Mammalian PRL activity relies on the formation of PRL trimers. LmPRL-1 might modulate the activity and function of its mammalian orthologs by physically interacting with them (3). Finally, a parasite-autonomous, intrinsic prosurvival effect of LmPRL-1 also needs to be envisaged (4).
