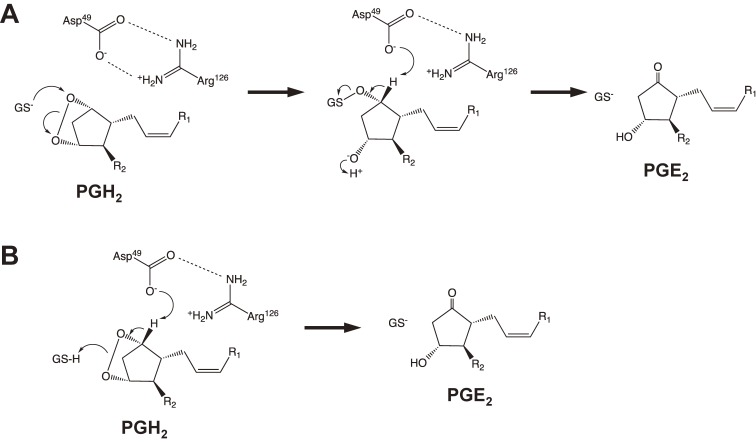
Figure 3.
Two suggested chemical mechanisms of GSH-dependent isomerization of PGH2 to PGE2 by mPGES-1.20) (A) The thiolate of GSH could be stabilized by Arg126 and could attack on the endoperoxide oxygen atom at the C-9 carbon of PGH2 to produce an unstable reaction intermediate. The subsequent proton abstraction at C-9 followed by S-O bond cleavages is mediated by Asp49, and then a carbonyl forms and the oxygen sulfur bond is broken to form PGE2. (B) The reaction starts by proton abstraction at C-9 via Asp49, and then a carbonyl forms and the endoperoxide is broken. The thiol of GSH functions as a proton donor to the developing C-11 oxyanion.