Abstract
It is difficult to distinguish the onset of renal function decline from the typical variation in estimated glomerular filtration rate (eGFR) measurements in clinical practice. In this study, we used data analysis incorporating smoothing techniques to identify significant trends despite large amounts of noise. We identified the starting points of meaningful eGFR decline based on eGFR trajectories. This was a retrospective observational study of 2533 type 2 diabetes patients. We calculated 1-year eGFR decline rates from the difference between each eGFR value and that of the previous year. We examined the prediction capacity of 1-year eGFR decline rate for renal prognosis. When we performed receiver operating characteristic analysis, the area under the curve of 1-year eGFR decline rate was 0.963 (95% confidence interval: 0.953–0.973). With a cut-off value of more than 7.5% eGFR decline during a 1-year period, the sensitivity was 98.8% and specificity was 82.3%. The predictive accuracy of 1-year eGFR decline rate for renal prognosis was high.
Keywords: renal outcome, type 2 diabetic nephropathy, change in eGFR
Introduction
Diabetic nephropathy is the leading cause of end-stage renal disease (ESRD), which is a threat to public health and a major financial burden for healthcare systems.1,2) In 2009, more than 871,000 people were treated for ESRD, and between 1980 and 2009, the prevalence of ESRD increased nearly 600%, from 290 to 1,738 cases per million in the United States. ESRD treatment costs the United States over $40 billion in public and private funds in 2009. The life expectancy of patients with ESRD has remained poor, and ESRD prevention is challenging (data from the web site of National Institute of Diabetes and Digestive and Kidney Diseases; https://www.niddk.nih.gov/health-information/health-statistics/Pages/kidney-disease-statistics-united-states.aspx. last accessed, September 28, 2016). The cardiovascular disease prognosis in patients with type 2 diabetes has markedly improved over the past 20 years, but the incidence of ESRD has decreased very little.3) Until the microalbuminuria stage, tight glycemic control can slow the progression of nephropathy. The Steno-2-study showed intensive treatment in patients with microalbuminuria could reduce cardiovascular disease events, all-cause mortality, and the need for dialysis.4,5) Thus, early interventional treatment for diabetic nephropathy is important.
Chronic kidney disease (CKD) was defined and classified in the Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines in 2002, with the aim of comprehensive disease recognition, early detection, providing treatment, and preventing or delaying renal disease progression while patients have normal renal function. CKD stages were classified based on glomerular filtration rate (GFR). Many studies have shown that increased urinary protein and albuminuria are associated with increased risk of ESRD.6,7) Worldwide cohort studies have confirmed that estimated GFR (eGFR) and albuminuria are independent risk factors for all-cause mortality, cardiovascular mortality, and risk of ESRD,8,9) and the Kidney Disease Improving Global Outcomes (KDIGO) classified the severity and stages of CKD based on GFR and proteinuria level in 2011. Diabetic nephropathy is one type of CKD, and a re-analysis by ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation) demonstrated that microalbuminuria was a risk factor for nephropathy progression and cardiovascular death.10,11)
GFR generally declines at a rate of 1 mL/min/year. However, patients who lose renal function faster than the average age-related decline in GFR tend to progress to ESRD. Krolewski et al. defined progressive renal decline as an eGFR loss of ⩾3.3% per year.12) They confirmed that progressive renal function decline often preceded the appearance of macroalbuminuria or microalbuminuria in type 1 diabetic patients. However, years of observation are required to firmly establish the presence of renal function decline, and a decline predictive of ESRD strongly depends on progression to macroalbuminuria. Given these considerations, the clinical value of determining GFR slopes in patients with normal urinary albumin excretion or microalbuminuria to identify progressive kidney disease is limited.13) Renal function worsens gradually in some patients, whereas sudden declines occur in others. The variations in eGFR decline patterns make it difficult for clinicians to identify when eGFR begins declining in each patient. Furthermore, eGFR fluctuates; for example, in a 60-year-old man with a serum creatinine of 0.7 mg/dL, a 0.1 mg/dL change in serum creatinine would result in a 13.6% change in eGFR (from 88.5 mL/min/1.73 m2 to 76.5 mL/min/1.73 m2). It is very difficult for clinicians to detect meaningful changes in eGFR due to this noise. In this study, we used data smoothing techniques to identify significant trends despite large amounts of noise and successfully identified starting points for meaningful eGFR decline based on eGFR trajectories.
Materials and methods
Study population.
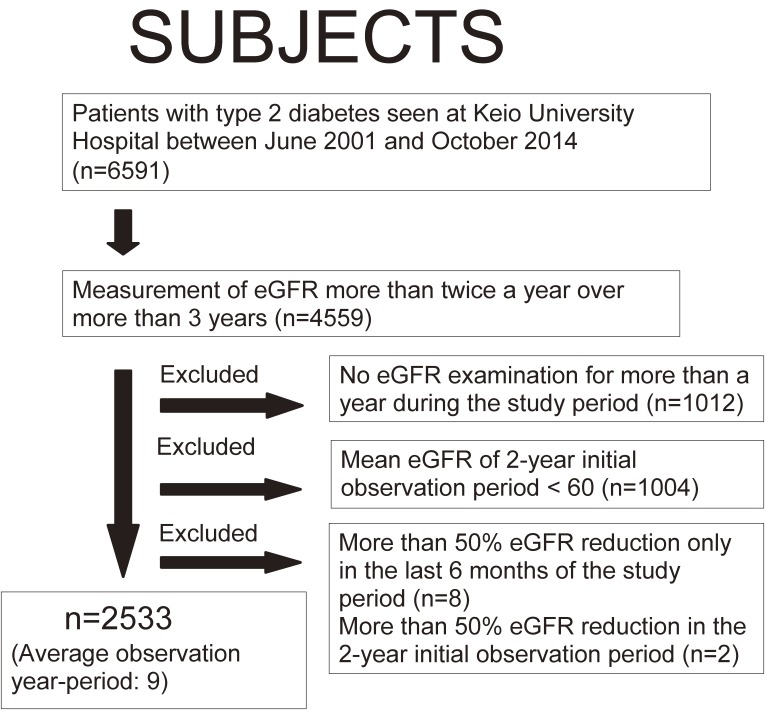
This was a single center-based retrospective cohort study. All type 2 diabetes patients seen in the Nephrology, Endocrinology, and Metabolism division of the Department of Internal Medicine at Keio University Hospital between June 2001 and October 2014 were candidates for this study (6591 patients, Fig. 1). Among these patients, subjects whose eGFR was measured more than twice in a half-year for more than 3 years were included in this study. Patients with no eGFR examination for more than 1 year during the study period, or a mean eGFR < 60 mL/min/1.73 m2 during the initial 2-year observation period were excluded. Patients with a more than 50% eGFR reduction in the last 6 months of the study period or more than 50% eGFR reduction in the initial 2-year observation period were also excluded. This study was approved by the Ethics Committee at the Keio University School of Medicine and conducted in accordance with the Declaration of Helsinki.
Figure 1.
Patient inclusion flow diagram. eGFR: estimated glomerular filtration rate.
Baseline assessment and measurements.
The study period included the initial 2-year observation period and the follow-up period thereafter. We collected blood test data from patients who visited the hospital for diabetes management every 1–3 months during the study period. All measurements were performed by the Department of Laboratory Medicine of Keio University Hospital using routine automated laboratory methods. Hemoglobin A1c (HbA1c) level was expressed in accordance with the National Glycohaemoglobin Standardization Program (NGSP) guidelines (%) as recommended by the Japanese Diabetes Society.14) For proteinuria, we replaced each urinary albumin/creatinine ratio (mg/g·Cr) with a class value (<30 mg/g·Cr = 1, ≥30–299 mg/g·Cr = 2, ≥300 mg/g·Cr = 3); we extracted the most frequent value from the initial 2-year observation period as albuminuria at baseline. We measured serum creatinine using an enzymatic method. eGFR was calculated using the formula established by the working group of the Japanese Chronic Kidney Disease Initiative15) as follows: eGFR (mL/min/1.73 m2) = 194 × (serum creatinine)−1.094 × (age)−0.287 (×0.739 for women). The number of eGFR measurements in the baseline period was 6.3 times/year ± 2.2, and that in the follow-up period was 5.5 times/year ± 1.6.
eGFR data smoothing.
We retrospectively analyzed the data of the patients who met the above criteria from June 2001 to October 2014. All available plasma creatinine values obtained during the follow-up period were used for analysis. We performed a smoothing technique to reduce the fluctuation in eGFR trajectory. The technique we used for data smoothing was a locally weighted processing method. Lowess (robust locally weighted regression and smoothing scatterplot) is a non-parametric regression method that combines multiple regression models in a k-nearest-neighbor-based meta-model.16) Cleveland described this method in 1979 to observe trends that are resistant to outliers, and its utility is highly accepted for the analysis of medical data that include many outliers.17) Loess is a developed version of Lowess.18)
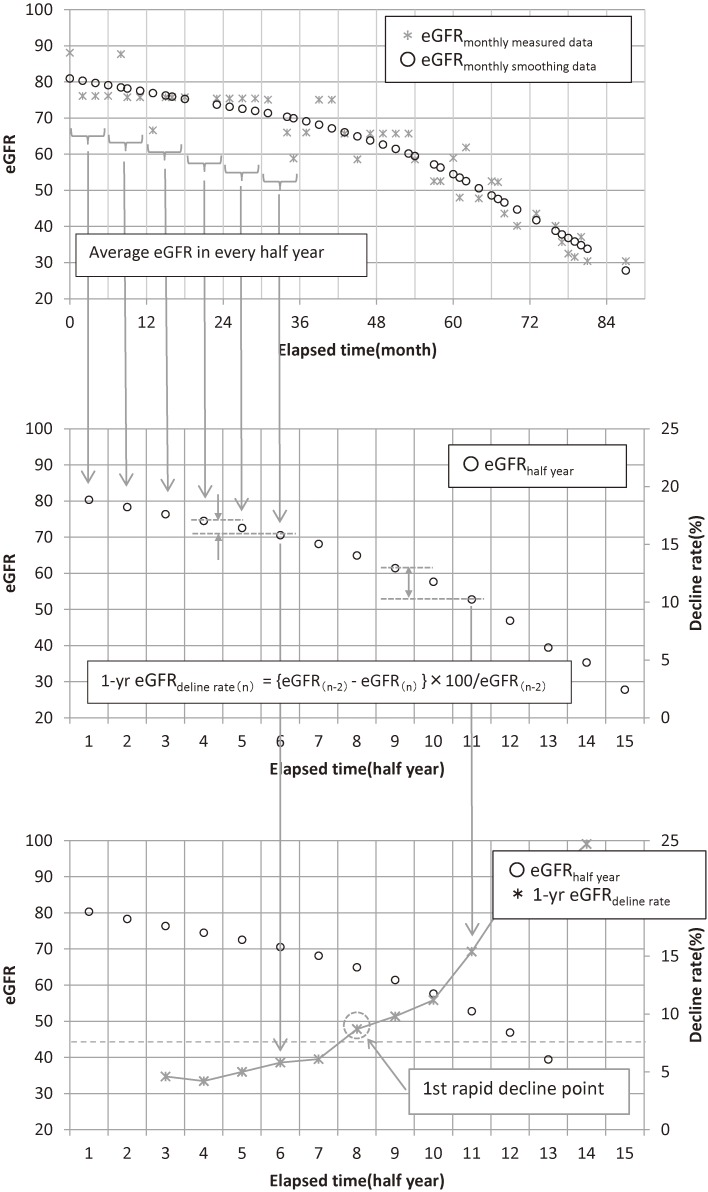
Conventional linear regression consists of finding the straight line in such a way so as to minimize the sum of the squared errors between the historical data points and the fitted linear equation. In the Loess method, a given local area is chosen to obtain a regression through the data points and to define a weight function, the value of which will be bigger around the center of the given area. This value will decrease as it gets closer to the edges of both sides and have a zero value outside the field. A regression value is obtained by placing the regression line so as to minimize the sum of the weighted distance between the regression line and given data points inside a given area. This smoothing method allows the isolation of trends reflecting both long-term and short-term variations only from given data points with non-parametric values, except for the neighboring outliers. We used the Loess function in the stats library of R version 3.2.2 (2015 The R Core Team), and we adopted 2/3 for span. At first, every eGFR value was smoothed using locally weighted processing (eGFRmonthly smoothing data) and average eGFRmonthly smoothing data for every half-year was calculated for each patient (eGFRhalf year). We calculated each 1-year eGFR decline rate from the difference between each eGFRhalf year value and that of the previous year (Fig. 2). We also used the maximum value of 1-year eGFR decline rate for receiver operating characteristic (ROC) analysis.
Figure 2.
How to calculate each 1-year eGFR decline rate from the difference between each eGFRhalf year value and that of the previous year. At first, every eGFR value was smoothed using locally weighted processing (eGFRmonthly smoothing data) [upper figure] and average eGFRmonthly smoothing data in every half-year was calculated for each patient (eGFRhalf year) [middle figure]. We calculated each 1-year eGFR decline rate from the difference between each eGFRhalf year value and that of the previous year [lower figure].
Outcomes.
The endpoint was defined as a decline in eGFRhalf year to less than half of the eGFRbaseline (the mean eGFR value in the initial 2-year observation period). Among subjects who met the endpoint, patients whose last eGFRhalf year decreased by less than half of eGFRbaseline as the result of rapid eGFR decline (greater than or equal to 30 mL per year during the last half year) were excluded because it was unclear whether their eGFR reduction was temporary or permanent.
Statistical analysis.
Statistical analysis was performed using SPSS statistical software (version 21.0; SPSS Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation (SD). The level of significance was set at p < 0.05. To analyze the predictive value of 1-year eGFR decline rate for the endpoint, we conducted ROC analysis by extracting the maximum 1-year eGFR decline rate value. We also used ROC analysis of the 2-year eGFR decline rate, 1-year eGFR decline rate without smoothing method, eGFRbaseline, proteinuria in the initial observation period, and proteinuria in the latest follow-up period to compare the predictive values of these factors. We also analyzed the cumulative incidence of renal endpoints in subjects whose 1-year eGFR decline rate was ≥7.5% with the Kaplan–Meier method examined using the log rank test.
Results
Clinical characteristics at baseline.
A total of 2533 patients with type 2 diabetes (age 59.4 ± 11 years; 36.4% women) were included in this study. The mean eGFR in the initial 2-year observation period was 77.1 ± 13 mL/min per 1.73 m2. The mean follow-up period was 9.1 ± 3.0 years. Patients’ clinical backgrounds in the initial 2-year observation period are shown in Table 1.
Table 1.
Clinical characteristics of 2533 type 2 diabetic patients. Data are expressed as mean ± standard deviation or as a percentage (%).
| Characteristic | |
|---|---|
| Average observation period (year) | 9.1 ± 2.9 |
| Female (%) | 36.4 |
| Age at baseline (year) | 59.4 ± 10.8 |
| eGFR (mL/min/1.73 m2) | 77.1 ± 13.0 |
| HbA1c (%) | 7.2 ± 1.1 |
| GA (%) | 20.1 ± 3.9 |
| Glu (mg/dl) | 148.4 + 33.7 |
| CPR (ng/ml) | 2.5 ± 1.6 |
| UA (mg/dl) | 5.4 ± 1.2 |
| HDL (mg/dl) | 53.9 ± 13.9 |
| LDL (mg/dl) | 117.4 ± 28.0 |
| TG (mg/dl) | 142.1 ± 89.6 |
| AST (IU/l) | 26.3 ± 12.5 |
| ALT (IU/l) | 29.5 ± 18.0 |
| Γ-GTP (U/l) | 53.8 ± 65.8 |
| CRP (mg/dl) | 0.6 ± 1.2 |
| HGB (g/dl) | 14.1 ± 1.4 |
| SBP (mmHg) | 135.7 ± 21.9 |
| DBP (mmHg) | 81.2 ± 13.7 |
| BMI (kg/m2) | 24.4 ± 4.4 |
| SBP ≧ 140 or DBP ≧ 90 mmHg or Antihypertensive drug usage (%) | 70.2 |
| BMI ≧ 25 kg/m2 (%) | 38.9 |
| Retinopathy (%) | |
| No apparent retinopathy | 85.6 |
| simple | 7.8 |
| pre proliferative or proliferative | 6.6 |
| Medication usage | |
| OHA (%) | 58.8 |
| Insulin (%) | 26.3 |
| ARB (%) | 49.8 |
| ACE-I (%) | 12.6 |
Outcomes.
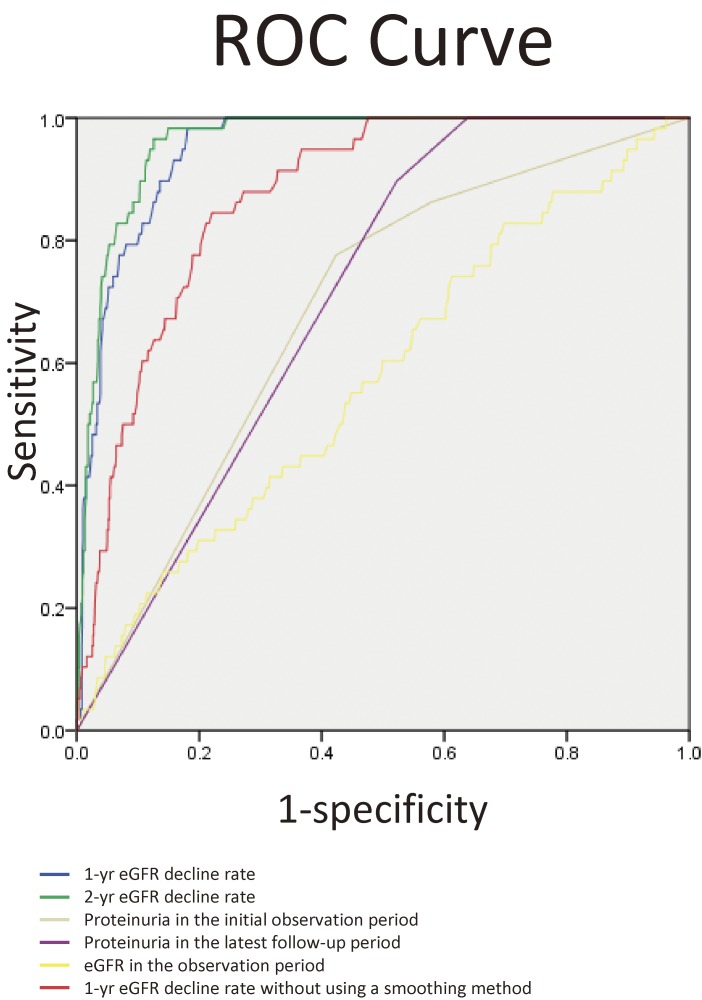
During the follow-up period, 85 (3.4%) patients reached the endpoint. When we performed ROC analysis for the endpoint, the area under the curve (AUC) of 1-year eGFR decline rate was 0.949 (95% confidence interval [CI]: 0.932–0.966). We determined a cut-off value for 1-year eGFR decline rate to identify subjects at high risk of the endpoint using ROC curves (Fig. 3). The cut-off value with the highest accuracy was 8.4% (sensitivity: 97.6%, its specificity: 86.0%). However, because we considered this diagnosis important for screening tests, we reduced the cut-off. We examined the predictive value of a reduced cut-off value of 7.5%, which was an arbitrary value, and found a sensitivity of 98.8% and specificity of 82.3% (Table 2). We performed a similar examination for the 2-year eGFR decline rate; the AUC value was 0.961 (95% CI: 0.947–0.975). The best cut-off value of the 2-year eGFR decline rate from the AUC was 15.8%. We examined the predictive value of a reduced cut-off value of 15%, and found its sensitivity was 96.5% and specificity was 89.4% (Table 3). We also performed ROC analysis of the 1-year eGFR decline rate without using a smoothing method. The AUC of the 1-year eGFR decline rate without using a smoothing method was 0.873 (95% CI: 0.839–0.908). We also performed ROC analysis for both proteinuria and mean eGFR during the initial 2-year observation period for the endpoint. The AUC of albuminuria during the initial 2-year observation period was 0.684 (95% CI: 0.620–0.748) and the AUC of mean eGFR in the initial 2-year observation period was 0.576 (95% CI: 0.500–0.652). For proteinuria, we also conducted ROC analysis of the latest proteinuria status during the follow-up period (Fig. 3). The AUC of the latest proteinuria in the follow-up period was 0.706 (95% CI: 0.657–0.755).
Figure 3.
ROC curves of different prediction markers for 50% decline in estimated glomerular filtration rate prediction. The AUC of 1-year eGFR decline rate was 0.949 (95% CI: 0.932–0.966). The AUC of 2-year eGFR decline rate was 0.961 (95% CI: 0.947–0.975). The AUC of albuminuria in the initial 2-year observation period was 0.684 (95% CI: 0.620–0.748). The AUC of albuminuria in the last observation period was 0.706 (95% CI: 0.657–0.755). The AUC of mean eGFR in the initial 2-year observation period was 0.576 (95% CI: 0.500–0.652).
Table 2.
Examination of cut-off value for 1-year estimated glomerular filtration decline rate
| Halves | Non-halves | |
|---|---|---|
| ≧7.5% (N = 517) | 84 | 433 |
| <7.5% (N = 2016) | 1 | 2015 |
| Sensitivity | 0.988 | |
| Specificity | 0.823 | |
Table 3.
Examination of cut-off value for 2-year estimated glomerular filtration decline rate
| Halves | Non-halves | |
|---|---|---|
| ≧15% (N = 342) | 82 | 260 |
| <15% (N = 2191) | 3 | 2188 |
| Sensitivity | 0.965 | |
| Specificity | 0.894 | |
We divided the study subjects into two groups based on whether their maximum 1-year eGFR decline rate was <7.5% or ⩾7.5%; the clinical background of each group is shown in Table 4. The average observation period was shorter in the group with a decline rate ⩾7.5%. Baseline high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and hemoglobin were lower in the group with a decline rate ⩾7.5%, and HbA1c, glucose, C-reactive protein, triglyceride, gamma-glutamyl transpeptidase, and albuminuria at baseline were higher in the group with a decline rate ⩾7.5% (Table 4). Diabetic retinopathy was significantly more frequent in the group with a decline rate ⩾7.5%. Body mass index and the prevalence of hypertension, which was defined as a systolic blood pressure ⩾140 or diastolic blood pressure ⩾90 mm Hg or the use of antihypertensive drugs, were not different between groups. Angiotensin receptor blockers were significantly more frequently prescribed in the group with a decline rate ⩾7.5%. Furthermore, the group with a decline rate ⩾7.5% used more oral hypoglycemic agents and insulin than did the group with a decline rate <7.5%.
Table 4.
Clinical characteristics of 2533 type 2 diabetic patients stratified by 1-year eGFR decline rate. Data for albuminuria (mg·g/Cr) are presented as percentage of each group.
| Characteristic | ≧7.5% (N = 517) | <7.5% (N = 2016) | P value |
|---|---|---|---|
| Average observation period (yr) | 7.4 ± 3.2 | 9.5 ± 2.7 | <0.001 |
| Female (%) | 39 | 35.8 | 0.16 |
| Age at baseline (yr) | 61.3 ± 12.0 | 59.9 ± 10.5 | 0.016 |
| Base eGFR (mL/min/1.73 m2) | 77.2 ± 13.7 | 77.1 ± 12.8 | 0.893 |
| HbA1c (%) | 7.4 ± 1.2 | 7.1 ± 1.0 | <0.001 |
| GA (%) | 20.5 ± 4.4 | 20.0 ± 3.7 | 0.072 |
| Glu (mg/dl) | 155.7 ± 39.4 | 146.5 ± 31.9 | <0.001 |
| CPR (ng/ml) | 2.7 ± l.8 | 2.4 ± 1.5 | 0.02 |
| UA (mg/dl) | 5.4 ± 1.3 | 5.4 ± 1.2 | 0.493 |
| HDL (mg/dl) | 52.2 ± 13.9 | 54.4 ± 13.9 | 0.002 |
| LDL (mg/dl) | 112.6 ± 27.8 | 119.0 ± 27.9 | 0.001 |
| TG (mg/dl) | 153.8 ± 95.5 | 139.1 ± 87.9 | 0.002 |
| AST (IU/l) | 27.8 ± 14.5 | 25.9 ± 12.0 | 0.007 |
| ALT (IU/l) | 30.2 ± 19.1 | 29.3 ± 18.0 | 0.336 |
| Γ-GTP (U/l) | 62.6 ± 76.6 | 51.5 ± 61.8 | 0.004 |
| CRP (mg/dl) | 0.9 ± 1.5 | 0.6 ± 1.1 | <0.001 |
| HGB (g/dl) | 13.7 ± 1.5 | 14.2 ± 1.3 | <0.001 |
| Albuminuria (%) | |||
| Lack of data (N = 1186) | 35 | 49.9 | |
| <30 mg·g/Cr (N = 621) | 19.5 | 25.8 | |
| 30–299 mg·g/Cr (N = 193) | 7.9 | 7.5 | |
| 300 mg·g/Cr< (N = 533) | 37.5 | 16.8 | |
| <0.001 | |||
| SBP (mmHg) | 135.6 ± 22.7 | 135.7 ± 21.7 | 0.909 |
| DBP (mmHg) | 79.4 ± 14.0 | 81.8 ± 13.5 | 0.006 |
| BMI (kg/m2) | 24.1 ± 4.7 | 24.4 ± 4.3 | 0.303 |
| SBP ≧ 140 or DBP ≧ 90 mmHg or Antihypertensive drug usage (%) | 70.2 | 70.2 | 0.513 |
| BMI ≧ 25 kg/m2 (%) | 35.6 | 40.1 | 0.295 |
| Retinopathy (%) | |||
| No apparent retinopathy | 73.8 | 88.6 | |
| simple | 12.6 | 6.6 | |
| pre proliferative or proliferative | 13.7 | 4.8 | |
| <0.001 | |||
| Medication usage | |||
| OHA (%) | 64.6 | 57.3 | 0.003 |
| Insulin (%) | 34.8 | 20 | <0.001 |
| ARB (%) | 59.6 | 47.3 | <0.001 |
| ACE-I (%) | 13.3 | 12.4 | 0.542 |
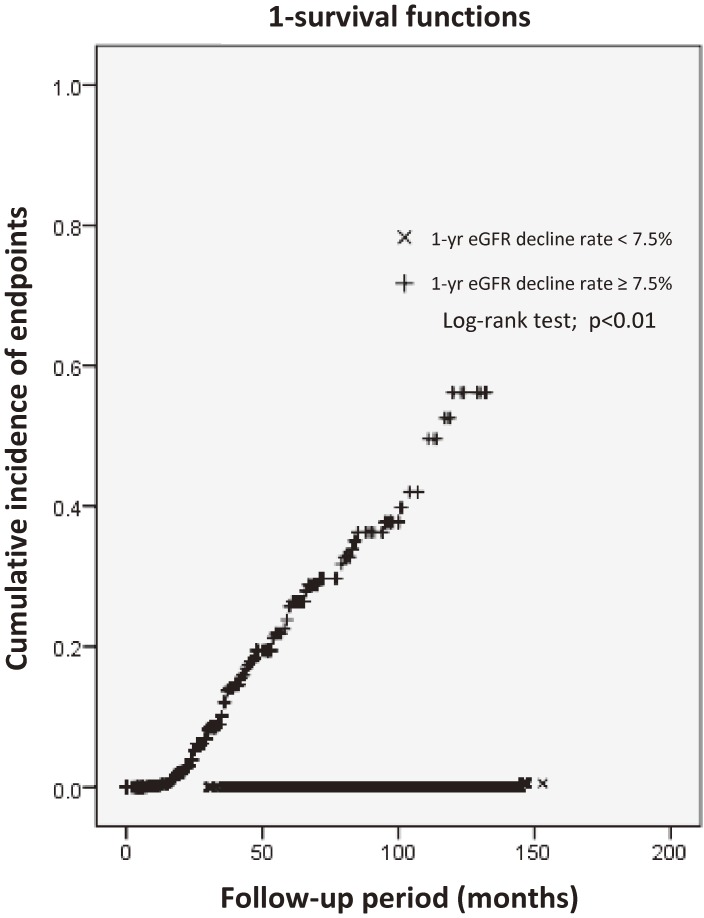
We also analyzed the cumulative renal endpoint incidence in subjects from the moment when their 1-year eGFR decline rate was ⩾7.5% with the Kaplan–Meier method (Fig. 4). The average survival period was 98 months (95% CI: 91.8–103.5), and only 1 (0.05%) patient whose 1-year eGFR decline rate was always <7.5% reached an endpoint outcome.
Figure 4.
Cumulative incidence of 50% decline in eGFR from the baseline value in patients with type 2 diabetes. eGFR, estimated glomerular filtration rate.
Discussion
Early renal function decline is observed in some diabetic patients, whereas in others the decline begins later. However, it has been difficult to predict the beginning of renal function decline. In this study, we elucidated that the predictive capacity of short-term eGFR decline rate for renal failure had good accuracy when the eGFR trajectory was smoothed. We also conducted ROC analyses for proteinuria and eGFR at baseline, which are the classical risk factors for renal failure, and found much better results with the eGFRmonthly smoothing data decline trajectory compared with baseline proteinuria and eGFR. It seems reasonable that the predictive power of eGFR decline is superior to that of conventional methods, because elapsed time is considered. To ensure the superior predictive power of our method, we performed ROC analyses for both the initial and the last proteinuria of the follow-up period. The results of both analyses were inferior to those of the 1-year eGFR decline rate. The clinical utility of the eGFRmonthly smoothing data trajectory is higher than eGFR, proteinuria, or clinical stage measured at a single time point. We evaluated the accuracy of the analysis by lowering the cut-off value to 7.5%; the sensitivity was 98.8% and the specificity was 82.3%. Thus, in patients with a 1-year eGFR decline rate of <7.5%, almost none reached the renal outcome during an average of 9 years of observation. In patients with a 1-year eGFR decline rate >7.5% at any point during follow-up, renal prognosis may be poor.
Most similar previous reports13,19,20) divided study subjects into two or three groups based on their eGFR decline rate during predetermined intervals and compared outcomes between groups. However, various transition patterns of eGFR decline exist. For example, a patient’s eGFR might have declined rapidly in the follow-up period after little decline in the first prefixed interval. Because conventional analysis methods cannot identify these various eGFR transition patterns, we cannot adopt the idea of rapid eGFR decline into our clinical judgment in practice. Moreover, eGFR fluctuates, and it is very difficult to identify a true eGFR trend with a large amount of fluctuating noise.
Our method using the eGFRmonthly smoothing data trajectory allowed us to pick up cases of rapid eGFR decline during disease progression. Because it allows us to identify the beginning of nephropathy progression in close to real time, the eGFRmonthly smoothing data trajectory makes it possible to treat patients at early stages. This method would be useful in real clinical practice.
A recent study using empagliflozin showed a 44% relative risk reduction in doubling of serum creatinine and a 39% reduction in incident or worsening nephropathy.21) New therapies to prevent renal function deterioration such as this increases the need to detect patients whose diabetic nephropathy is prone to progress. It is also necessary to examine whether therapy is effective for patients with progressive eGFR decline.
Our study had some limitations. Because most subjects were simultaneously being treated and prescribed medications by a general physician or cardiologist, the medication data were incomplete. In this study, we selected patients with baseline average eGFRs of ≥60 mL/min/1.73 m2, because subjects who already had decreased renal function were inevitably susceptible to reach the endpoint. It is not clear if our eGFRmonthly smoothing data trajectory method can also predict renal prognosis in subjects with eGFRs < 60 mL/min/1.73 m2. When the cut-off value of eGFR rate of decline is set at 7.5%, the specificity is 82.3%, thus the false positive rate was still high. To reduce false positives and improve the prediction accuracy for renal function prognosis, it is better to also measure the 2-year eGFR decline rate. When the cut-off value was set at 15% for the 2-year eGFR decline rate, the sensitivity was 0.965 and the specificity was 89.4%, which showed that specificity was improved. The merit of the 1-year measurement was that renal function decline can be detected earlier. The combined use of 1- and 2-year eGFR decline rates may increase the prognostic prediction accuracy in patients identified with progressive eGFR decline.
Jerums et al. concluded the optimal method for accurately estimating an early decline in GFR, from a normal to subnormal level, is yet to be defined.22) We revealed early progressive eGFR declines from eGFR trajectories.
Conclusions
We examined the prediction capacity of 1-year eGFR decline rate for renal prognosis. When we performed ROC analysis, the AUC of 1-year eGFR decline rate was 0.949 (95% CI: 0.932–0.966). With a cut-off value of a >7.5% decline in eGFR during a 1-year period, the sensitivity was 98.8% and the specificity was 82.3%. The predictive accuracy of 1-year eGFR decline rate for renal prognosis was high compared with other indicators. In cases where the 1-year eGFR decline rate is ever >7.5%, the prognosis may be poor.
Acknowledgements
This work was supported by funding from Asahi Kasei Corporation. J.N. and S.M. collected the data and wrote the manuscript. M.F. and N.O. performed the statistical analyses. T.K. and H.I. contributed to the discussion and reviewed and edited the manuscript.
Abbreviations
- ACE-I
angiotensin-converting enzyme inhibitor
- ADVANCE
Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation
- ALT
alanine aminotransferase
- ARB
angiotensin II receptor antagonists
- AST
aspartate aminotransferase
- AUC
area under the curve
- BMI
body mass index
- CI
confidence interval
- CKD
chronic kidney disease
- CPR
C-peptide immunoreactivity
- CRP
C-reactive protein
- DBP
diastolic blood pressure
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- GA
glycated albumin
- GFR
glomerular filtration rate
- Glu
glucose
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- HGB
hemoglobin
- KDIGO
Kidney Disease Improving Global Outcomes
- KDOQI
Kidney Disease Outcomes Quality Initiative
- LDL
low-density lipoprotein
- NGSP
National Glycohaemoglobin Standardization Program
- OHA
oral hypoglycemic agents
- ROC
receiver operating characteristic
- SBP
systolic blood pressure
- SD
standard deviation
- TG
triglyceride
- UA
uric acid
- γ-GTP
gamma-glutamyl transpeptidase
References
- 1).Arefzadeh A., Lessanpezeshki M., Seifi S. (2009) The cost of hemodialysis in Iran. Saudi J. Kidney Dis. Transpl. 20, 307–311. [PubMed] [Google Scholar]
- 2).Beladi Mousavi S.S., Sametzadeh M., Hayati F., Fatemi S.M. (2010) Evaluation of acquired cystic kidney disease in patients on hemodialysis with ultrasonography. Iran. J. Kidney Dis. 4, 223–226. [PubMed] [Google Scholar]
- 3).Gregg E.W., Li Y., Wang J., Burrows N.R., Ali M.K., Rolka D., Williams D.E., Geiss L. (2014) Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 370, 1514–1523. [DOI] [PubMed] [Google Scholar]
- 4).Gaede P., Vedel P., Larsen N., Jensen G.V., Parving H.H., Pedersen O. (2003) Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 348, 383–393. [DOI] [PubMed] [Google Scholar]
- 5).Gaede P., Lund-Andersen H., Parving H.H., Pedersen O. (2008) Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 358, 580–591. [DOI] [PubMed] [Google Scholar]
- 6).Ishani A., Grandits G.A., Grimm R.H., Svendsen K.H., Collins A.J., Prineas R.J., Neaton J.D. (2006) Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J. Am. Soc. Nephrol. 17, 1444–1452. [DOI] [PubMed] [Google Scholar]
- 7).Iseki K., Ikemiya Y., Iseki C., Takishita S. (2003) Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 63, 1468–1474. [DOI] [PubMed] [Google Scholar]
- 8).Matsushita K., van der Velde M., Astor B.C., Woodward M., Levey A.S., de Jong P.E., Coresh J., Gansevoort R.T. (2010) Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohort; a collaborative meta-analysis. Lancet 375, 2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Gansevoort R.T., Matsushita K., van der Velde M., Astor B.C., Woodward M., Levey A.S., de Jong P.E., Coresh J., the Chronic Kidney Disease Prognosis Consortium (2011) Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative metaanalysis of general and high-risk population cohorts. Kidney Int. 80, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Ninomiya T., Perkovic V., de Galan B.E., Zoungas S., Pillai A., Jardine M., Patel A., Cass A., Neal B., Poulter N., Mogensen C.E., Cooper M., Marre M., Williams B., Hamet P., Mancia G., Woodward M., Macmahon S., Chalmers J., the ADVANCE Collaborative Group (2009) Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. 20, 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Wada T., Haneda M., Furuichi K., Babazono T., Yokoyama H., Iseki K., Araki S., Ninomiya T., Hara S., Suzuki Y., Iwano M., Kusano E., Moriya T., Satoh H., Nakamura H., Shimizu M., Toyama T., Hara A., Makino H., the Research Group of Diabetic Nephropathy, Ministry of Health, Labour, and Welfare of Japan (2014) Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes. Clin. Exp. Nephrol. 18, 613–620. [DOI] [PubMed] [Google Scholar]
- 12).Krolewski A.S., Niewczas M.A., Skupien J., Gohda T., Smiles A., Eckfeldt J.H., Doria A., Warram J.H. (2014) Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care 37, 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Pavkov M.E., Knowler W.C., Lemley K.V., Mason C.C., Myers B.D., Nelson R.G. (2012) Early renal function decline in type 2 diabetes. Clin. J. Am. Soc. Nephrol. 7, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Seino Y., Nanjo K., Tajima N., Kadowaki T., Kashiwagi A., Araki E., Ito C., Inagaki N., Iwamoto Y., Kasuga M., Hanafusa T., Haneda M., Ueki K. (2010) Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 1, 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., Yamagata K., Tomino Y., Yokoyama H., Hishida A. (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992. [DOI] [PubMed] [Google Scholar]
- 16).Cleveland W.S. (1979) Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 74, 829–836. [Google Scholar]
- 17).Kawanishi M., Nakamoto A., Hiraoka M., Kuroki Y., Itoh M., Takata K., Horiuchi I., Konemori G., Amioka H., Matsuura H., Kajiyama G. (1989) Indices for prediction of coronary sclerosis with particular reference to Apo-B/Apo-AI ratio. J. Jpn. Atheroscler. Soc. 17, 939–947. [Google Scholar]
- 18).Cleveland W.S., Devlin S.J. (1988) An approach to regression analysis by local fitting. J. Am. Stat. Assoc. 83, 596–610. [Google Scholar]
- 19).Turin T.C., Coresh J., Tonelli M., Stevens P.E., de Jong P.E., Farmer C.K., Matsushita K., Hemmelgarn B.R. (2012) Short-term change in kidney function and risk of end-stage renal disease. Nephrol. Dial. Transplant. 27, 3835–3843. [DOI] [PubMed] [Google Scholar]
- 20).Coresh J., Turin T.C., Matsushita K., Sang Y., Ballew S.H., Appel L.J., Arima H., Chadban S.J., Cirillo M., Djurdjev O., Green J.A., Heine G.H., Inker L.A., Irie F., Ishani A., Ix J.H., Kovesdy C.P., Marks A., Ohkubo T., Shalev V., Shankar A., Wen C.P., de Jong P.E., Iseki K., Stengel B., Gansevoort R.T., Levey A.S., the CKD Prognosis Consortium (2014) Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311, 2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Wanner C., Inzucchi S.E., Lachin J.M., Fitchett D., von Eynatten M., Mattheus M., Johansen O.E., Woerle H.J., Broedl U.C., Zinman B., for the EMPA-REG OUTCOME Investigators (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375, 323–334. [DOI] [PubMed] [Google Scholar]
- 22).Jerums G., Ekinci E.I., Panagiotopoulos S., MacIsaac R.J. (2012) Early glomerular filtration rate loss as a marker of diabetic nephropathy. European Endocrinology. 8, 27–31. [Google Scholar]