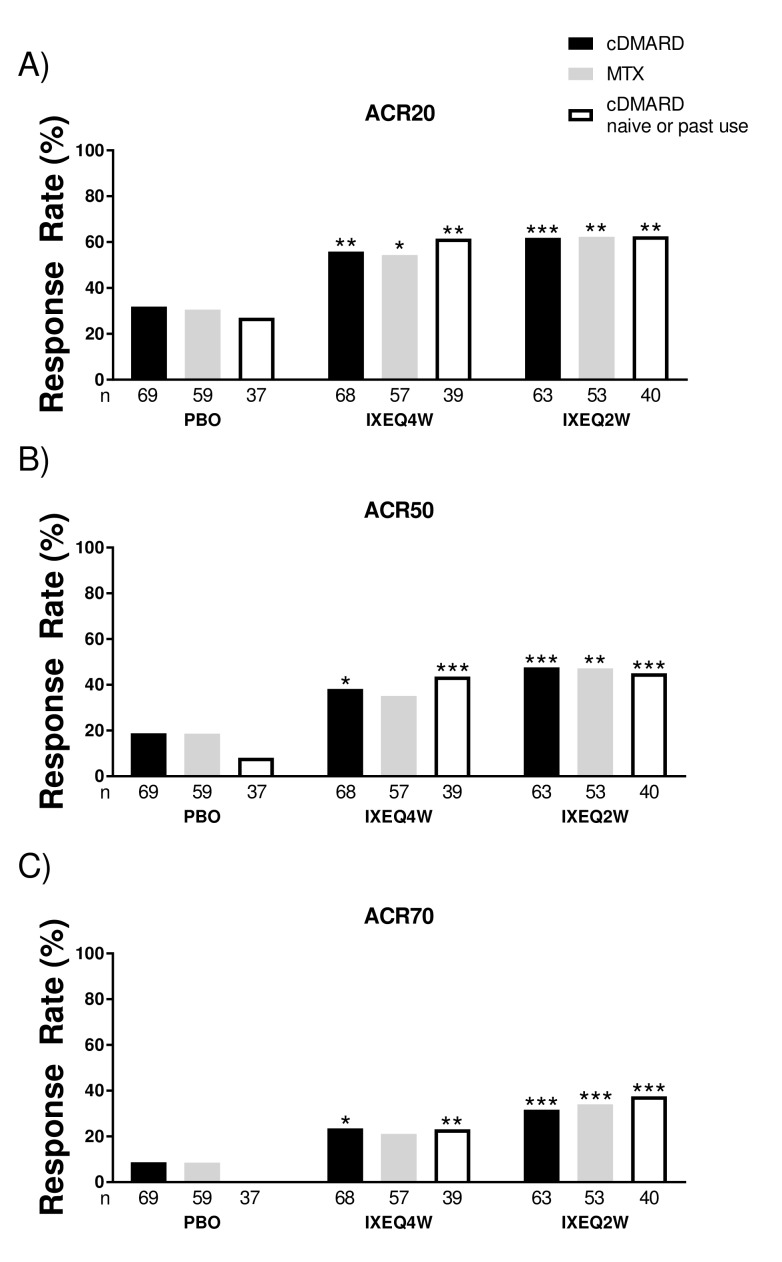
Figure 1.
ACR response rates at 24 weeks in patients treated with PBO, IXEQ4W or IXEQ2W alone or in combination with cDMARDs or MTX. The proportions of patients achieving ACR20 (A), ACR50 (B) and ACR70 (C) are shown. The study was not designed to test equivalence or non-inferiority of treatment with ixekizumab alone versus treatment with ixekizumab in combination with cDMARDs. ACR20/50/70, 20%/50%/70% American College of Rheumatology response; cDMARD, conventional disease-modifying antirheumatic drugs; PBO, placebo; IXEQ4W, 80 mg ixekizumab once every 4 weeks; IXEQ2W, 80 mg ixekizumab once every 2 weeks; n, number of patients; MTX, methotrexate. *P<0.05, **P<0.01, ***P<0.001, all versus PBO.