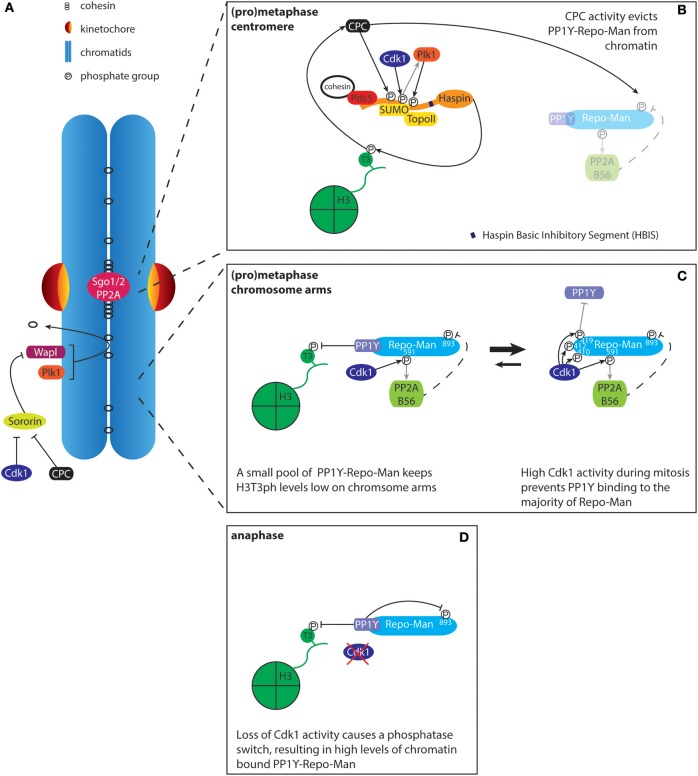
Figure 3.
Regulation of histone H3T3 phosphorylation by Haspin and PP1Y-Repo-Man. (A) The prophase pathway removes cohesin from the chromosome arms. Sororin binds to the cohesin complex and this interaction is required for maintaining stable cohesion. During mitosis, Plk1 phosphorylates the cohesin subunit SA2 while Cdk1 and Aurora B phosphorylate Sororin. This results in the release of Sororin from cohesin, leading to the Wapl dependent removal of cohesin from chromatin. Centromeres are protected against the prophase pathway through recruitment of Sgo1/2-PP2A. The recruitment of Sgo1-PP2A results in de-phosphorylation of SA2 and Sororin, rendering the centromeric cohesin complexes resistant to Wapl activity. Effectively, this results in the concentration of cohesin/Pds5A/B and thus Haspin at centromeres, thereby contributing to the defined localization of the CPC at the inner centromere. (B) The cohesin-associated protein Pds5A/B, in conjunction with SUMOylated Topoisomerase II (TopoII), recruits Haspin to the inner centromere. Haspin phosphorylation by Aurora B (CPC), Cdk1, and Plk1 releases HBIS dependent Haspin auto-inhibition. Phosphatase activity toward H3T3ph by PP1Y-Repo-Man is inhibited through phosphorylation of Repo-Man by the CPC, which prevents Repo-Man recruitment to chromatin. (C) At the chromosome arms, Haspin levels are lower, most likely due to reduced levels of cohesin and SUMOylated TopoII. Low levels of chromatin targeted PP1Y-Repo-Man are sufficient to maintain H3T3 in a dephosphorylated state. (D) Upon anaphase onset, loss of Cdk1 activity promotes the PP1Y-Repo-Man interaction, resulting in high levels of the active complex associated with chromatin.