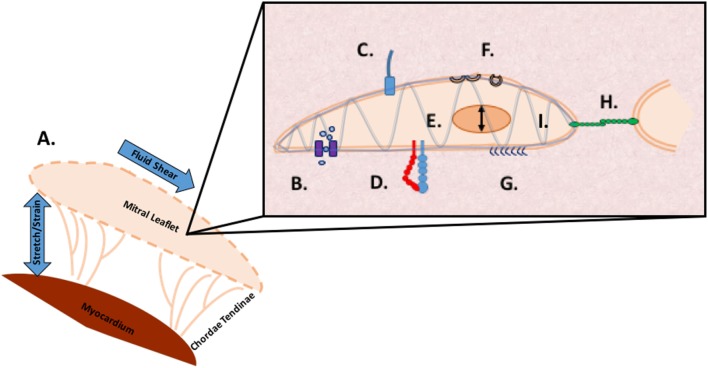
Figure 4.
Methods of mechanosensing in the mitral valve. A. At a global level, the valve is subjected to flexure as the valve opens, shear as the blood flows through the valve, flexure as the valve closes, and tension as the valve seals shut to prevent regurgitation. At a microscopic level, mechanotransduction converts these extracellular forces into intracellular signaling through multiple cellular apparatuses. B. Mechano-sensitive ion channels convert mechanical force exerted on the cell membrane into electrical or biochemical signals. C. The axoneme of primary cilia convert extracellular cues into various signaling pathways as well as coupling transduction with voltage-gated channels D. Integrins are the main receptors connecting the cytoskeleton to the extracellular matrix (ECM) and transmit mechanical stress across the plasma membrane E. In nuclear deformation physical force is transmitted across the nuclear envelope to the nuclear interior where they modulate gene expression from physical deformation of genetic material F. Caveolae flatten into the plasma membrane when stimulated by cell-surface tension, relieving tension and physically sequestering proteins, growth hormones, and cytokines G. The glycocalyx transmits fluid shear stress to the cell through core proteins which connect to the actin cytoskeleton and cell membrane mediating cell signaling H. Cadherins are cell adhesion proteins that create zipper like structures at cell junctions to maintain stable intercellular adhesion and mechanical coupling between cells and the adherens junction to transform mechanical to chemical signals as well as interacting with integrins through actin filaments I. Directly or indirectly, the load bearing cytoskeleton is common to the various mechanosensing modalities. Often clustering at focal adhesions, the cytoskeleton rapidly transmits ECM stimulus into cellular response through actin filament reorganization.