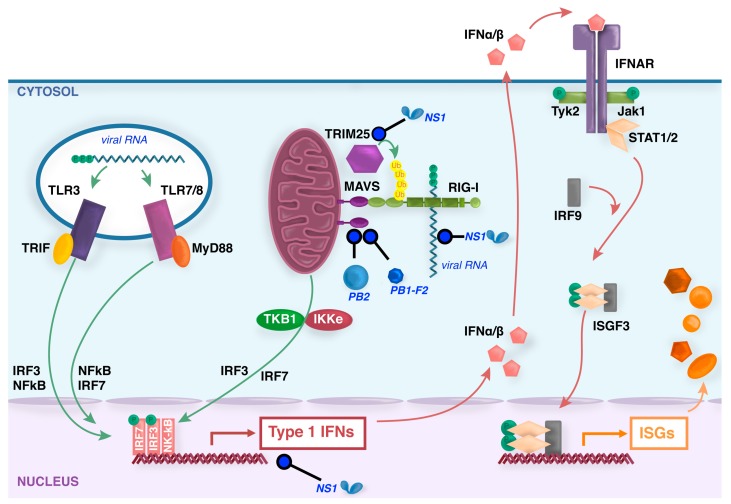
Figure 1.
Induction of type I interferons (IFNs) by influenza viruses. During entry of influenza virus into some host cells, virions within the endosome expose viral RNA to toll-like recepotrs (TLR)3/7/8. Virus replication produces triphosphorylated vRNA and potentially dsRNA by-products that are recognized by the ubiquitously expressed cytoplasmic sensor retinoic acid inducible gene (RIG)-I. TLR signaling by the adaptors TIR-domain-containing adapter-inducing interferon-β (TRIF) or myeloid differentiation factor (MyD)88, and RIG-I signaling by the adaptor mitochondrial antiviral signaling (MAVS), trigger signal transduction cascades that result in activation of IFN regulatory factor (IRF)3/7 and NF-κB factors that translocate to the nucleus to induce synthesis of type I IFN mRNAs. Secreted type I IFNs signal through the IFN-α/β receptor complex (IFNAR) via activation of the intracellular kinases Jak1 and Tyk2, which phosphorylate the signal transducer and activator of transcription (STAT) transcription factors that, together with IRF-9, form the interferon-stimulated gene factor 3 (ISGF3) that translocates to the nucleus and activates the transcription of ISGs. In virus-infected cells, the nonstructural (NS)1 protein binds to and sequesters dsRNA to antagonize RIG-I activation, as well as interacting with various host proteins to inhibit transcription, processing or nuclear export of cellular mRNAs [20,21]. NS1 also binds to tripartite motif-containing (TRIM)25 protein and prevents essential ubiquitination of RIG-I [22]. Viral PB1-F2 [23] and PB2 proteins [24,25] inhibit MAVS function in the mitochondria.