Table 1.
Small molecules that were repurposed for the treatment of SMA.
| Name | Structure | Mode of Action | MWa | cLogPb | PSAc | HBAd | HBDe | Ref |
|---|---|---|---|---|---|---|---|---|
| Sodium butyrate (1) |
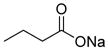
|
Weak class I HDAC inhibitor | 110 | 0.6 | 26 | 2 | 1 | 39 |
| Sodium phenylbutyrate (2) |
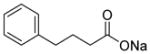
|
Weak class I HDAC inhibitor | 186 | 2.0 | 26 | 2 | 1 | 40,45–49 |
| Valproic Acid (3) |
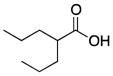
|
Weak class I HDAC inhibitor | 144 | 2.8 | 37 | 2 | 1 | 41,50–54 |
| Suberoylanilide hydroxamic acid (4) |
|
Potent HDAC class I and II | 264 | 1.0 | 78 | 5 | 3 | 42,43 |
| Trichostatin A (5) |
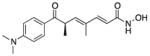
|
Potent HDAC class I and IIb | 302 | 1.9 | 70 | 5 | 2 | 44,55,56 |
| Riluzole (6) |
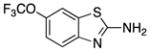
|
Neuro-protective | 234 | 3.2 | 48 | 4 | 2 | 57–62 |
| Hydroxyurea (7) |
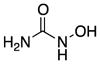
|
Increases Nitric Oxide | 76 | −1.8 | 75 | 4 | 4 | 63–66 |
| Ceftriaxone (8) |
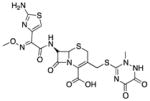
|
Unknown | 554 | 0.02 | 209 | 18 | 5 | 67–69 |
| Albuterol (9) |
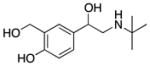
|
β2 Adrenergic Receptor Agonist | 239 | 0.06 | 73 | 4 | 4 | 70–74 |
| Aclarubicin (10) |
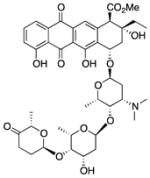
|
Antibiotic | 812 | 3.4 | 217 | 16 | 4 | 75, 76 |
MW, molecular weight;
cLogP, calculated LogP;
PSA, polar surface area;
HBA, hydrogen-bond acceptor;
HBD, hydrogen-bond donor.
