FIGURE 1.
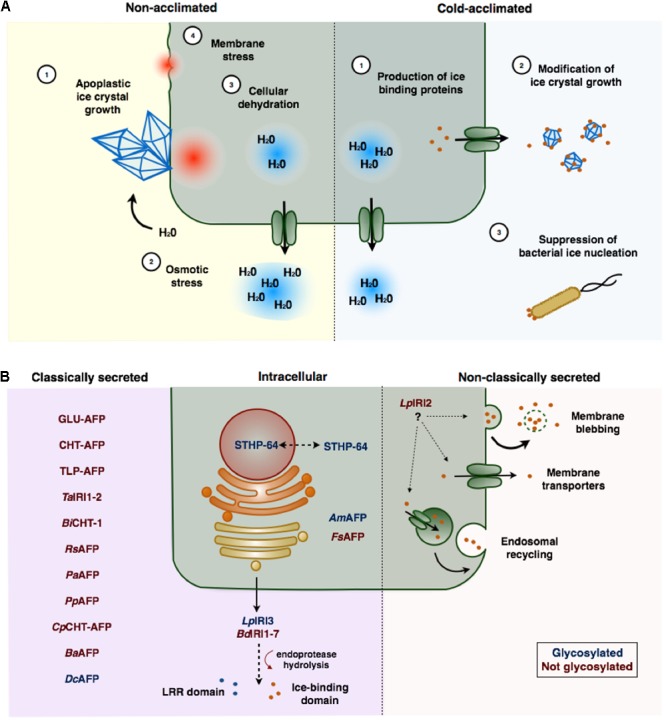
(A) Freezing stress and ice-binding proteins (IBP)-induced freeze protection in plants. In the absence of IBPs, large ice crystals form in the apoplast that can physically damage plasma membranes (1). As water molecules join the ice crystal lattice an osmotic gradient is formed (2) resulting in the sequestration of intracellular water, and consequent cellular dehydration (3). The loss of cell volume may cause cells to collapse or rupture (4). Cold acclimation induced expression of IBPs, which are typically secreted to the apoplast (1), adsorb to seed ice crystals preventing their growth (2). IBPs may also prevent freezing associated with bacterial ice nucleation (3). (B) Localization and post-translational modification of ice-binding proteins in plants. Most plant IBPs contain amino-terminal secretion signals and are localized to the apoplast through the endoplasmic reticulum (ER)–Golgi apparatus pathway. Some of these proteins (LpIRI3 and BdIRI1-7), appear to be hydrolyzed following secretion, releasing an LRR domain from the ice-binding domain. LpIRI2, which lacks a signal peptide, is secreted, likely through a non-classical secretion pathway. Few IBPs have been localized to the intracellular space. Glycosylated proteins are indicated in red, proteins that are not glycosylated are indicated in blue. IBPs are named according to Table 1.
