Figure 1. Nucleotide Biosynthesis.
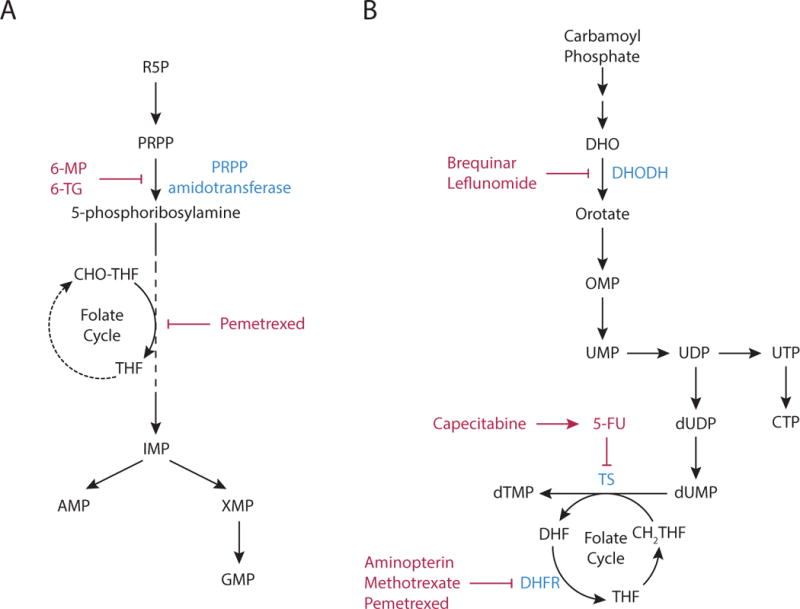
(A) Purine nucleotide synthesis. The first reaction in purine production generates 5-phosphoribosyl-1-pyrophosphatae (PRPP) from ribose 5-phosphate (R5P). The second step is catalyzed by PRPP amidotransferase, and commits PRPP to purine synthesis. This step can be inhibited by the antimetabolites, 6-mercaptopurine (6-MP) and 6-thioguanine (6-TG). Subsequent steps in the pathway assemble the purine ring and result in the formation of inosine monophosphate (IMP), which in turn can be converted to either adenosine monophosphate (AMP) or guanosine monophosphate (GMP) by distinct reactions. The synthesis of the purine ring requires N10-formyl-tetrahydrofolate (CHO-THF) via a reaction that can be inhibited by pemetrexed.
(B) Pyrimidine nucleotide synthesis. Pyrimidine nucleotide synthesis begins with the conversion of carbamoyl phosphate to the pyrimidine base orotate. One of the steps in pathway is catalyzed by dihydroorotate dehydrogenase (DHODH), which can be inhibited by brequinar sodium and leflunomide. Next, orotate is combined with PRPP to generate orotate monophosphate (OMP), which is subsequently converted to uridine monophosphate (UMP). UMP can be phosphorylated to form UDP and UTP, and the latter can be further converted to citidine triphosphate (CTP). Uridine nucleotides can also be used for de novo thymine nucleotide synthesis. UDP is converted to deoxy-UMP (dUMP), and the enzyme thymidylate synthase (TS) generates dTMP by catalyzing the methylation of dUMP using N5,N10-methylene-tetrahydrofolate (CH2-THF) as the methyl donor. TS activity is inhibited by the antipyrimidine 5-fluorouracil (5-FU) and the 5-FU pro-drug capecitabine. Thymidine synthesis can also be inhibited by the antifolates aminopterin, methotrexate, and pemetrexed, as these drugs inhibit the enzyme dihydrofolate reductase (DHFR), limiting the availability of CH2-THF.
