Abstract
Background:
Although indoor residual spraying (IRS) is an effective tool for malaria control, its use contributes to high insecticide exposure in sprayed communities and raises concerns about possible unintended health effects.
Objective:
The Venda Health Examination of Mothers, Babies and their Environment (VHEMBE) is a birth cohort study initiated in 2012 to characterize prenatal exposure to IRS insecticides and exposures’ impacts on child health and development in rural South Africa.
Methods:
In this report, we describe the VHEMBE cohort and dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) serum concentrations measured in VHEMBE mothers when they presented for delivery. In addition, we applied a causal inference framework to estimate the potential reduction in population-level p,p′-DDT and p,p′-DDE serum concentrations under five hypothetical interventions. A total of 751 mothers were enrolled.
Results:
Serum concentrations of p,p′ isomers of DDT and DDE were above the limit of detection (LOD) in of the samples, whereas the o,p′ isomers were above the LOD in at least 80% of the samples. Median (interquartile range) p,p′-DDT and p,p′-DDE serum concentrations for VHEMBE cohort participants were 55.3 (19.0–259.3) and 242.2 (91.8–878.7) ng/g-lipid, respectively. Mothers reporting to have lived in a home sprayed with DDT for malaria control had times higher p,p′-DDT and p,p′-DDE serum concentrations than those who never lived in a home sprayed with DDT. Of the five potential interventions tested, we found increasing access to water significantly reduced p,p′-DDT exposure and increasing the frequency of household wet mopping significantly reduced p,p′-DDT and p,p′-DDE exposure.
Conclusion:
Our findings suggest that several intervention approaches may reduce DDT/DDE exposure in pregnant women living in IRS communities. https://doi.org/10.1289/EHP353
Introduction
In 2015, malaria infected approximately 214 million people worldwide and resulted in nearly 438,000 deaths (World Health Organization 2015). Indoor residual spraying (IRS), the application of insecticides to interior walls, ceilings, and eaves, is a malaria-vector control policy adopted by 88 countries (World Health Organization 2014b), protecting approximately 116 million people worldwide (World Health Organization 2015). The World Health Organization’s (WHO) Pesticide Evaluation Scheme recommends 12 insecticides from four chemical classes for IRS that include organochlorine (OC), organophosphate, carbamate, and pyrethroid insecticides (World Health Organization 2014a). Although banned in most countries, at least 10 countries, including Botswana, Democratic Republic of Congo, Gambia, India, Mozambique, Namibia, South Africa, Swaziland, Zambia, and Zimbabwe, used the OC insecticide dichlorodiphenyltrichloroethane (DDT) for IRS in 2014 (World Health Organization 2014b). The comparative advantages of DDT to other insecticides used for IRS include its longer residual efficacy () (World Health Organization 2014a) and noncontact spatial repellent properties (Grieco et al. 2007). In some areas of South Africa, such as the Limpopo Province, DDT had been continuously used since the 1940s (Mabaso et al. 2004).
Although the benefits of decreased malaria infection are clear (Kim et al. 2012; Mabaso et al. 2004), the use of DDT for malaria control has contributed to uniquely high DDT exposure in sprayed communities (Aneck-Hahn et al. 2007; Bouwman et al. 2006; Channa et al. 2012; Ortiz-Pérez et al. 2005; Sereda et al. 2009; Van Dyk et al. 2010; Whitworth et al. 2014). Of particular concern is exposure to pregnant women, as DDT can cross the placental barrier and expose the developing fetus (Waliszewski et al. 2000). Biomonitoring studies of pregnant women living in IRS areas are sparse, but Channa et al. (2012) reported median p,p′-DDT and p,p′-dichlorodiphenyldichloroethylene (DDE) plasma concentrations in women delivering in a high-risk malaria area of KwaZulu-Natal Province in South Africa () to be 2,788 and -lipid, respectively. These levels are substantially higher than median p,p′-DDT and p,p′-DDE plasma concentrations in South African women giving birth in areas of low malaria risk (; p,p′-DDT = 27 and p,p′-DDT = 184 ng/g-lipid) and in nonmalarial areas ( p,p′-DDT = 7 and p,p′-DDE = 26 ng/g-lipid) (Channa et al. 2012).
The few studies that have investigated determinants of DDT exposure in populations living in IRS areas have found that living in either a home or village sprayed for malaria control was associated with higher DDT body burden (Aneck-Hahn et al. 2007; Bouwman et al. 2006; Channa et al. 2012; Herrera-Portugal et al. 2005; Manaca et al. 2011; Sereda et al. 2009; Van Dyk et al. 2010; Whitworth et al. 2014), but few additional determinants have been examined. Whitworth et al. (2014) recently reported that, in a subset of Limpopo women living in unsprayed villages (), women with water piped into their yards had 73% and 61% lower DDT and DDE levels, respectively, than had women whose water source was a public tap. In addition, Limpopo women living in DDT-sprayed homes () who performed more than six preventative measures to prepare their home for IRS (e.g., covering food/water, taking furniture out of the house) had 40% lower DDT serum levels than women who performed fewer than four preventative measures (Whitworth et al. 2014). In the only study investigating the determinants of prenatal exposure to DDT in an IRS population (), Channa et al. (2012) found that length of breastfeeding, age, parity, level of education, and permanent employment of the mothers were inversely associated with p,p′-DDT/E plasma concentrations.
Most studies have quantified determinants of xenobiotic exposures by fitting a single regression model with all covariates and interpreting the coefficients as the association of each variable with serum levels. This approach may not give valid estimates of effects and inference if the model inputs are not a priori specified or improper assumptions about the relationship between the exposure and outcome are made (e.g., linear relationship) (Ritter et al. 2014). Further, covariates along the causal pathway between exposure and outcome (mediators) are often improperly included within a single model, biasing the results (Schisterman et al. 2009). Public health researchers are ultimately interested in the marginal (population-level) effect of specific interventions on exposure. Under a causal inference framework, one can test the population change in insecticide levels that would be observed if specified interventions were implemented (e.g., increasing access to water, cleaning floors at a given frequency) (Pearl 2000). Therefore, the results of a causal inference analysis are tailored to the ultimate purpose of the study — instituting public health policies that reduce insecticide exposure in IRS communities.
In our study, we investigated p,p′/o,p′-DDT and p,p′/o,p′-DDE serum concentrations of pregnant women enrolled in the Venda Health Examination of Mothers, Babies and their Environment (VHEMBE) study. We examined bivariate determinants of DDT and DDE exposure and evaluated five hypothetical interventions aiming to reduce exposure using a causal inference framework.
Methods
Study Population
VHEMBE is a birth cohort study based in the rural Vhembe district of South Africa’s Limpopo Province. The study aims to investigate the potential effects of IRS insecticide exposure on child growth and development. Between August 2012 and December 2013, we enrolled mother–newborn dyads at the time of maternal presentation for delivery at Tshilidzini Hospital. Eligible women were , spoke Tshivenda at home, lived within 20 km of the hospital, planned to remain in the area, had not been diagnosed with malaria during pregnancy, had contractions apart, and gave birth to a viable singleton. We obtained informed consent by verbally explaining the study procedures prior to the collection of study data. All human subject protocols were approved by institutional review boards at the University of California, Berkeley; McGill University; the University of Pretoria; the Limpopo Department of Health and Social Development; and Tshilidzini Hospital.
Out of the 1,649 women approached to participate in the VHEMBE study, 920 were eligible (). Of those eligible, 152 refused enrollment (), 14 did not complete a baseline questionnaire (), and three did not provide a sufficient blood sample for DDT analysis (). In total, 751 mothers completed a baseline questionnaire and provided a blood sample, and 722 were visited at their homes by our staff one week after delivery (). On average, mothers enrolled in the VHEMBE study were 1.6 years younger and had given birth to 0.2 fewer children prior to the index child than had eligible mothers who refused enrollment ().
Maternal and Home Characteristics
Tshivenda-speaking study staff administered a baseline questionnaire before hospital discharge to collect data on demographic characteristics (e.g., maternal age, primary language, marital status, education, and household income), parity, length of cumulative breastfeeding, hygiene/cleaning habits, and housing and IRS-use history. Household income was compared with the food poverty line determined by Statistics South Africa (W. Ruch, written communication, May 2014; Statistics South Africa 2014). We also assessed nutrient intake by administering a quantitative food frequency questionnaire (FFQ) validated in the Limpopo population (MacIntyre et al. 2001a,b; MacIntyre et al. 2001c). FFQ parameters were generated using the Food Finder 3 program (Nutritional Intervention Research Unit and Biomedical Research Division). Maternal height was measured using a wall-mounted stadiometer (Charder HM210D; Taichung City, Taiwan), and weight was measured with a digital scale (Beurer PS06; Ulm, Germany). All measurements were performed in triplicate, with the mean values used to calculate body mass index (BMI). At the one-week visit, we performed home inspections to collect information on household water source, building type, and homes’ latitude and longitude coordinates.
Generating Spatial Variables
We used 2009 Spot 5 satellite imagery to create spatial variables to test for the association between location and p,p′-DDT/E serum concentrations. We calculated the minimum distance from each participant’s home to the nearest body of water using ArcGIS’s ‘Near’ tool. Water bodies were defined based on publicly accessible national datasets but were supplemented with manual additions, drawn using ArcScan based on the Spot 5 imagery. The distance-to-body-of-water variable was created based on the hypothesis that participant homes located near bodies of water (potential mosquito habitats) would be more likely to undergo IRS applications and proximity would result in higher exposure to participants. In addition, we used the kernel density ArcTool to calculate the number of structures per hectare within 250 and 1,000 m buffers from the participant’s home. Our structure density variable was created based on the hypothesis that density of IRS use in the area (spraying is done by structure) would influence the exposure of the participant within that area. This variable was generated using ArcScan to extract imagery pixels with a radiometric resolution of 220 or higher. The resulting extracted pixel layer was cleaned by hand (to minimize misclassification of other features such as roads and structures). The pixels were then converted to points, and the kernel density was completed.
Measurement of p,p′ and o,p′ Isomers of DDT/E
Maternal blood was collected into red-top vacutainer tubes by study nurses prior to delivery () or immediately after delivery (). Samples were immediately processed and stored at . Serum aliquots were sent on dry ice to Emory University’s Rollins School of Public Health for the measurement of p,p′ and o,p′ DDT/E using gas chromatography-tandem mass spectrometry (GC-MS) with isotope dilution quantification (Barr et al. 2003). The limit of detection (LOD) and limit of quantification (LOQ) for p,p′-DDT, o,p′-DDT, and o,p′-DDE were 0.01 and , respectively. For p,p′-DDE, the LOD and LOQ were 0.03 and , respectively. Total lipid concentrations were estimated based on triglycerides and total cholesterol concentrations (Phillips et al. 1989), measured using standard enzymatic methods (Roche Chemicals, Indianapolis, IN). Quality-control procedures included field spikes, field blanks, matrix-matched calibrants, and laboratory-prepared serum and reagent blanks analyzed concurrently with participants’ samples. The Supplemental Information (SI) describes the laboratory method used to quantify the p,p′ and o,p′ isomers of DDT/E and provides detailed quality control information.
Data Analysis
We used Spearman’s correlation or Kruskal-Wallis tests to examine the bivariate relationships of participant characteristics and potential determinants of exposure with DDT/DDE serum concentrations. Only the p,p′ isomers of DDT and DDE were considered for these analyses due to lower detection frequencies in the o,p′ isomers of DDT and DDE. For the p,p′-DDT/E serum concentrations below the laboratory’s LOQ, but above the LOD, we assigned those values the GC-MS machine-read value ( for p,p′-DDT and for p,p′-DDE). For p,p′-DDT serum concentrations below the laboratory’s LOD (), we imputed those values from maximum likelihood estimates of the lognormal distribution of the detected serum values (Lubin et al. 2004). Spearman’s correlation tests were also used to examine the correlation between p,p′-DDT and p,p′-DDE serum concentrations. Associations were considered statistically significant if were .
For the causal inference analysis, we aimed to estimate the marginal geometric mean difference in p,p′-DDT/E serum levels (Y) if, contrary to fact, all VHEMBE mothers were given an intervention () relative to a scenario in which none of the mothers were given that intervention (): , where is a matrix of covariates. In addition, we tested the effect of potential interventions by whether the mother reported ever living in a home sprayed with DDT to explore exposure reduction effect modification by spray status. Covariates used in the TMLE analysis included the following: if the mother ever lived in a home sprayed with DDT for malaria control (, categorical); if the mother lived in a home sprayed with DDT for malaria control during pregnancy (, categorical); the frequency of IRS in the home where the mother lived during pregnancy (, ordinal); if the mother lived in a village sprayed for malaria control during pregnancy (, categorical); the frequency of IRS in the village where the mother lived during pregnancy (, ordinal); the time spent in an IRS home (, no. of years); the mother’s age at delivery (, years); the education level of mother at delivery (, ordinal); household income (, Rands per household member per month); whether the pregnancy home was a rondavel with earthen walls and thatched roof (, categorical); parity of mother at delivery (, no. of previous births); breastfeeding history (, no. of months); presence of a rondavel on homestead (, categorical); if the household owned livestock (, categorical); proximity of mother’s home to the nearest body of water (, meters); structure density within 250-m radius of the mother’s home (, no./hectare); and maternal BMI after delivery ().
The potential interventions that we evaluated included: 1) living in a home with piped water [)]; 2) living in a home in which floors were mopped more than seven times weekly (median frequency reported by mothers) [)], 3) washing bed sheets more than two times per month (median frequency reported by mothers) [)]; 4) avoiding a high-fat diet () [)]; and 5) avoiding local dairy/meat/poultry fish products during pregnancy [)]. The potential interventions for this analysis were selected because they may be modifiable characteristics that were hypothesized to reduce DDT/DDE exposure, while maintaining effective malaria control.
The marginal geometric mean difference of p,p′-DDT/DDE serum concentrations for each intervention was evaluated in separate models using targeted maximum likelihood estimations (TMLE), a doubly robust substitution estimator that generates unbiased estimates if either models for the estimation of the exposure or determinant mechanisms are correct (Rose and van der Laan 2011; van der Laan 2006; van der Laan and Rubin 2006). A directed acyclic graph (DAG) was generated to conceptualize the estimation of serum levels and interventions and to identify potential confounders (Figure S1) (Textor et al. 2011). Missing covariate values () were imputed at random based on their observed probability distributions.
To estimate and , we used the Super Learner algorithm, an ensemble machine learning algorithm that uses a weighted combination of algorithms to return a prediction function that minimizes cross-validated mean squared error (van der Laan et al. 2007). We assessed positivity using the propensity score for each intervention and found that our positivity assumption holds for all interventions as the lowest propensity score was 0.07, and the median propensity scores across all interventions ranged from 0.53 to 0.75 (Table S1). To estimate and , we used the Super Learner algorithm with the following candidate algorithms: generalized linear models, generalized additive models, Bayesian linear model, support vector machine, recursive partitioning and regression trees, elastic net, neural network, local polynomial regression, and random forest. The associated weights used by Super Learner for estimating and are presented in the Supplemental Material (Tables S2 and S3). We used bootstrapping () to estimate 95% confidence intervals (CI) based on the percentile method (Efron 1979). Data analyses were performed using the statistical programs R (version 3.1.3; R Development Core Team) and ArcGIS (version 10.3; ESRI Corporation).
Exposure Levels Comparison with Other Populations
We compared VHEMBE lipid-adjusted p,p′-DDT and p,p′-DDE serum concentrations to serum/plasma levels previously reported in 1) adults living in IRS communities and 2) pregnant women from non-IRS communities in the United States. The median and inter-quartile ranges (IQR) were used to compare serum/plasma concentrations across studies, as those descriptive statistics were the most commonly reported. Because Aneck-Hahn et al. (2007) reported only the arithmetic mean and standard deviation (SD) of men living and not living in DDT-sprayed homes, the geometric mean (GM) and geometric standard deviation (GSD) were estimated according to equations presented in Jean and Helms (1983). We sampled 1,000 values from a log-normal distribution using the estimated GM and GSD to estimate the median and IQR of population from Aneck-Hahn et al. (2007). As only wet-weight concentrations (ng/mL) were presented by Whitworth et al. (2014), the Study of Women and Babies (SOWB) researchers graciously provided the lipid-adjusted distributions for comparison (K.W. Whitworth, written communication, October 2014). We compare only the Van Dyk et al. (2010) results for p,p′-DDE in adults living in home sprayed days prior to blood collection because the detection frequency for p,p′-DDT in sprayed homes (5%) and p,p′-DDT/E in unsprayed communities were low (0 and 33%, respectively). Only the lipid-adjusted values from the control group () were used from the case-control study of Bhatia et al. (2004). The p,p′-DDT/E serum concentrations from pregnant women who participated in the 1999–2000, 2001–2002, and 2003–2004 National Health and Nutrition Examination Study (NHANES) (DDT , DDE ) were combined (Center for Disease Control 2000, 2002, 2004). In the three NHANES surveys, p,p′-DDE was detected in 100% of the samples, and p,p′-DDT was detected in 37% of the samples .
Results
Study Participants
All mothers were born in South Africa and were black Africans. They had a mean (SD) age of 24.9 (6.3) years at delivery (Table 1). Most of the mothers had less than a 12th-grade education (54.9%), lived below the South African food poverty line of per person per month (58.3%), and were multiparous (56.8%). Almost a third (31.4%) of the mothers reported living in a village sprayed for malaria control during pregnancy, 3.1% reported living in a home sprayed with DDT during pregnancy, and 33.8% reported living in a home sprayed with DDT for malaria control in their lifetime. Of those mothers reporting that their home was sprayed for malaria control during pregnancy (), the majority reported that they were inside the home during IRS (80%) and did not move household items outside prior to IRS (58%). However, 68% did report that they covered household items prior to IRS.
Table 1.
Demographic characteristics of participants in the VHEMBE study, Limpopo, South Africa ().
| Characteristic | (%)a | |
|---|---|---|
| Maternal age (years) | ||
| 18–24 | 377 | (50.2) |
| 25–30 | 172 | (22.9) |
| 30–35 | 111 | (14.8) |
| 91 | (12.1) | |
| Primary language | ||
| Tshivenda | 734 | (97.7) |
| Tshitsonga (Xitsonga) | 14 | (1.9) |
| Tshipedi (Sepedi) | 3 | (0.4) |
| Married or living as married | ||
| No | 392 | (52.2) |
| Yes | 359 | (47.8) |
| Education | ||
| 412 | (54.9) | |
| Completed grade 12 | 229 | (30.5) |
| Further studies started | 50 | (6.7) |
| Diploma or further degree | 60 | (8.0) |
| Povertyb | ||
| Above food poverty line | 310 | (41.3) |
| Below food poverty line | 438 | (58.3) |
| Don’t know | 3 | (0.4) |
| Parity | ||
| 0 | 325 | (43.3) |
| 1 | 201 | (26.8) |
| 225 | (30.0) | |
| Mother ever had malaria | ||
| No | 727 | (96.8) |
| Yes | 24 | (3.2) |
Percentages may not add to 100% due to rounding.
Food poverty line based on Statistics South Africa (370 Rands or about monthly income per household member) (W. Ruch, written communication, May 2014; Statistics South Africa 2014).
Serum Concentrations of DDT and DDE
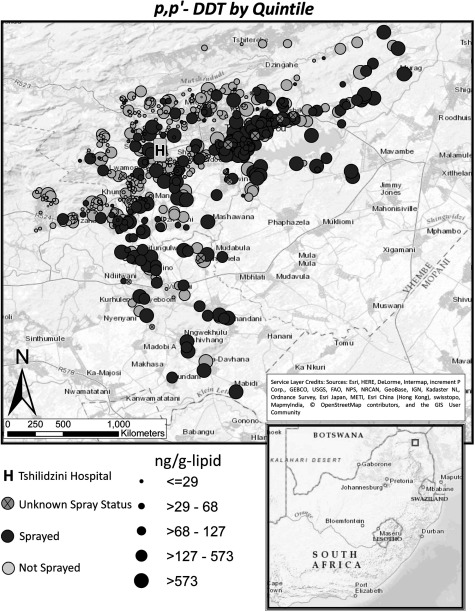
Serum concentrations were typically above the LOD for all DDT/DDE isomers (), with p,p′-DDT and p,p′-DDE above the LOD in 98% and 100% of the samples, respectively. Furthermore, p,p′-DDT/E concentrations were above the LOQ in of the samples and o,p′ DDT/E were above the LOQ in of the samples (Table 2). Median (IQR) p,p′-DDT and p,p′-DDE concentrations were and , respectively. Extreme serum concentration outliers were observed for both p,p′-DDT () and p,p′-DDE (). VHEMBE mothers’ p,p′-DDT and p,p′-DDE serum concentrations were strongly correlated (, ). Exposure to p,p′-DDT and p,p′-DDE was elevated among women living south and west of Tshilidzini Hospital (Figures 1 and S2).
Table 2.
DDT and DDE serum concentrations in VHEMBE participants, Limpopo, South Africa (ng/g-lipid).
| Isomer | a | b | GMc | GSDc | Min | 10th% | 25th% | Median | 75th% | 90th% | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|
| p,p′-DDT | 98.0 | 90.7 | 69.6 | 6.7 | 8.1 | 19.0 | 55.3 | 259.3 | 946.2 | 15027.6 | |
| o,p′-DDT | 90.5 | 66.6 | - | - | 7.1 | 22.6 | 72.0 | 2029.3 | |||
| p,p′-DDE | 100.0 | 97.2 | 287.9 | 4.8 | 44.7 | 91.8 | 242.2 | 878.7 | 2577.7 | 26301.3 | |
| o,p′-DDE | 82.7 | 48.2 | - | - | 6.9 | 13.0 | 117.5 |
. LOD for p,p′-DDT, o,p′-DDT, and o,p′-DDE was and the LOD for p,p′-DDE was 0.03 .
. LOQ for p,p′-DDT, o,p′-DDT, and o,p′-DDE was and the LOQ for p,p′-DDE was .
, . GM and GSD not calculated for o,p′ isomers due to lower detection frequencies. For p,p′-DDT/E, GM and GSD calculations include values below the LOD using imputed values from maximum likelihood estimates of the lognormal distribution and values below the LOQ, but above the LOD, using GC/MS machine-read values.
Figure 1.
Spatial distribution of p,p'-DDT concentrations in relation to Tshilidzini Hospital.
Bivariate Determinants Analysis
Mothers reporting that the villages in which they lived during pregnancy were sprayed for malaria control every year () had significantly higher () p,p′-DDT and p,p′-DDE serum concentrations than mothers who lived in an unsprayed village had () (median p,p′-DDT: 562.7 vs. -lipid; median p,p′-DDE: 1,431.1 vs. -lipid, respectively) (Table 3 and S4). Mothers who lived in a home that was sprayed with DDT during their pregnancy () had median p,p′-DDT and p,p′-DDE concentrations an order of magnitude higher than those who did not () (p,p′-DDT: 736.9 vs. -lipid; p,p′-DDE: 2,129.0 vs. -lipid, respectively).
Table 3.
p,p′-DDT and p,p′-DDE concentrations (ng/g-lipid) by selected characteristics in VHEMBE participants, Limpopo, South Africa.
| Exposure characteristic | %a | p,p′-DDT | p,p′-DDE | |||||
|---|---|---|---|---|---|---|---|---|
| Median | IQRb | Median | IQRb | p-Valuec | ||||
| Frequency of IRS in villaged,e | ||||||||
| Never | 516 | (68.7) | 38.1 | (16.0–131.5) | 178.1 | (73.1–549.5) | ||
| Some years | 136 | (18.1) | 105.4 | (32.9–374.9) | 498.0 | (129.8–1322.5) | ||
| Most years | 26 | (3.5) | 156.4 | (54.1–588.7) | 606.2 | (216.5–1744.4) | ||
| Every year | 63 | (8.4) | 562.7 | (128.4–1463.7) | 1431.1 | (318.9–3119.9) | ||
| Don’t know | 1 | (0.1) | 44.2 | (44.2–44.2) | 72.4 | (72.4–72.4) | ||
| Refused | 9 | (1.2) | 153.0 | (22.7–935.9) | 652.2 | (46.5–2742.4) | ||
| Village sprayed for malaria control during pregnancyd | ||||||||
| No | 488 | (65.0) | 37.1 | (16.0–130.3) | 178.1 | (73.5–527.1) | ||
| Yes | 236 | (31.4) | 181.4 | (41.0–679.0) | 604.0 | (172.9–1960.3) | ||
| Don’t know | 27 | (3.6) | 52.2 | (19.6–131.0) | 168.1 | (63.0–834.7) | ||
| Frequency of IRS in homed,e | ||||||||
| Never | 560 | (74.6) | 40.5 | (16.2–137.6) | 180.2 | (76.0–549.5) | ||
| Some years | 106 | (14.1) | 152.5 | (37.4–460.7) | 565.2 | (154.0–1573.1) | ||
| Most years | 20 | (2.7) | 268.8 | (61.2–736.2) | 873.6 | (287.8–2115.4) | ||
| Every year | 59 | (7.9) | 574.5 | (240.6–1503.2) | 1851.9 | (387.7–3300.5) | ||
| Don’t know | 1 | (0.1) | 44.2 | (44.2–44.2) | 72.4 | (72.4–72.4) | ||
| Refused | 5 | (0.7) | 935.9 | (22.7–1322.1) | 2742.4 | (46.5–3627.3) | ||
| Home ever sprayed with DDT for malaria controld | ||||||||
| No | 478 | (63.6) | 33.5 | (14.8–106.5) | 155.8 | (68.7–439.4) | ||
| Yes | 254 | (33.8) | 225.5 | (55.5–733.4) | 803.6 | (238.3–2189.2) | ||
| Don’t know | 19 | (2.5) | 68.4 | (33.2–407.3) | 204.9 | (72.4–1452.3) | ||
| Home sprayed with DDT for malaria control during pregnancyd | ||||||||
| No | 720 | (95.9) | 50.0 | (18.6–236.9) | 230.9 | (87.2–803.3) | ||
| Yes | 23 | (3.1) | 736.9 | (161.8–1726.7) | 2129.0 | (840.4–3238.1) | ||
| Don’t know | 8 | (1.1) | 183.5 | (80.2–493.8) | 332.8 | (191.4–1535.5) | ||
| Water source | 0.01 | |||||||
| Water not piped into home | 322 | (42.9) | 82.6 | (24.9–342.6) | 321.4 | (102.3–1249.9) | ||
| Water piped into home | 400 | (53.3) | 43.3 | (15.8–219.7) | 194.3 | (80.8–674.5) | ||
| Lost to follow-up | 29 | (3.9) | 48.9 | (30.9–162.5) | 197.1 | (67.1–938.1) | ||
| Number of times per week household wet mops | ||||||||
| 344 | (45.8) | 83.0 | (25.1–368.9) | 328.7 | (123.0–1247.3) | |||
| 377 | (50.2) | 40.1 | (16.0–191.1) | 180.7 | (77.3–640.6) | |||
| Don’t know | 30 | (4.0) | 53.0 | (31.9–154.9) | 193.8 | (68.4–915.9) | ||
| Number of times per month mother’s bed sheets are washed | 0.71 | 0.89 | ||||||
| 309 | (41.1) | 51.9 | (17.0–246.6) | 268.4 | (86.2–963.7) | |||
| 410 | (54.6) | 58.0 | (20.2–260.1) | 239.4 | (96.7–755.7) | |||
| Don’t know | 32 | (4.3) | 53.0 | (30.3–264.9) | 218.4 | (71.1–1153.6) | ||
| Mother consumed local animal products during pregnancyf | ||||||||
| No | 519 | (69.1) | 45.0 | (17.1–220.2) | 211.3 | (76.9–793.6) | ||
| Yes | 232 | (30.9) | 82.6 | (27.6–359.4) | 333.2 | (119.2–1232.2) | ||
| Fat consumed during pregnancy | 0.47 | 0.45 | ||||||
| (75th percentile) | 563 | (75.0) | 58.3 | (19.6–278.9) | 277.3 | (93.3–969.5) | ||
| (75th percentile) | 188 | (25.0) | 49.6 | (18.6–203.4) | 201.6 | (87.7–776.5) | ||
Percentages may not add to 100% due to rounding.
.
from Kruskall-Wallis tests for all determinants except for fat consumption ( from Spearman’s correlation test).
Spray information reported by mother.
Refers to the village and home the mother lived during her pregnancy.
Animal products include meat, poultry, eggs, dairy, and fish.
Parity and breastfeeding history were associated with p,p′-DDE serum concentrations but not p,p′-DDT concentrations (Table S4). Mothers who had water piped into their home, wet mopped at least seven times a week, did not consume locally raised animal products, did not own livestock, lived farther from a body of water, and did not have a rondavel (traditional South African homes with earthen walls and thatched roofs) on their homestead had significantly lower p,p′-DDT and p,p′-DDE serum concentrations ().
Causal Inference Intervention Analysis
We estimated that a population in which everyone wet mopped their floors at least seven times per week (median frequency reported by VHEMBE mothers) would have marginal geometric mean p,p′-DDT and p,p′-DDE serum concentrations 14.6 (95% CI: , ) and 69.0 (95% CI: , ) ng/g-lipid, respectively (Table 4), lower than in a population where everyone wet mopped their floors fewer than seven times per week. In addition, a population in which everyone had piped water would have marginal GM p,p′-DDT and p,p′-DDE serum concentrations 20.0 (95% CI: , ) and 50.2 (95% CI: , 2.0) ng/g-lipid, respectively, lower than a population in which everyone’s water supply was not on their homestead. However, this reduction was only significant for p,p′-DDT concentrations. There was suggestive evidence that not consuming local animal products during pregnancy reduced p,p′-DDT and p,p′-DDE serum concentrations, but the results were not statistically significant. Finally, we found that washing bed sheets at an increased frequency and not eating a high fat diet did not significantly reduce DDT or DDE exposures.
Table 4.
Estimation of the marginal geometric mean difference in p,p′-DDT and p,p′-DDE concentrations (ng/g-lipid) from five hypothetical interventions.a
| Population | Interventions | p,p′-DDT | p,p′-DDE | ||||
|---|---|---|---|---|---|---|---|
| Unexposed () | Exposed () | b | 95% CI | 95% CI | |||
| All | Water not piped into home | Water piped into home | 722 | (, ) | () | ||
| Wet mopping home c | Wet mopping home c | 721 | (, ) | (, ) | |||
| Washing bed sheets c | Washing bed sheets c | 719 | 11.2 | (, 25.5) | 19.5 | (, 66.8) | |
| Eating a high fat dietd | Not eating a high fat dietd | 751 | 0.3 | (, 14.5) | 30.2 | (, 90.7) | |
| Consuming local animal products during pregnancye | Not consuming local animal products during pregnancye | 751 | (, 3.1) | (, 17.7) | |||
| Home ever sprayed with DDT | Water not piped into home | Water piped into home | 245 | (, ) | (, ) | ||
| Wet mopping home c | Wet mopping home c | 245 | (, ) | (, ) | |||
| Washing bed sheets c | Washing bed sheets c | 245 | 41.7 | (, 322.9) | 60.6 | (, 938.5) | |
| Eating a high fat dietd | Not eating a high fat dietd | 254 | 4.0 | (, 238.5) | 44.0 | (, 784.0) | |
| Consuming local animal products during pregnancye | Not consuming local animal products during pregnancye | 254 | (, 106.2) | (, 219.5) | |||
| Home never sprayed with DDT | Water not piped into home | Water piped into home | 461 | (, 33.2) | (, 180.1) | ||
| Wet mopping home c | Wet mopping home c | 460 | (, 12.4) | (, 36.8) | |||
| Washing bed sheets c | Washing bed sheets c | 458 | 4.3 | (, 37.5) | (, 92) | ||
| Eating a high fat dietd | Not eating a high fat dietd | 478 | 0.4 | (, 42.8) | 25.6 | (0.3, 208.9) | |
| Consuming local animal products during pregnancye | Not consuming local animal products during pregnancye | 478 | (, 41.2) | (, 189.2) | |||
parameter estimate for the marginal geometric mean difference in serum concentrations from TMLE; 95% confidence interval estimated from 1,000 bootstrapped estimates of the observed data using the percentile method (Efron 1979).
Participants excluded if answering “Don’t know” to question, refusing to answer question, or lost to follow up.
Cleaning intervention levels are the median frequencies reported by mothers.
High fat diet defined as %ile of fat intake for all mothers.
Animal products include meat, poultry, eggs, dairy, and fish.
When we explored for effect modification, increasing access to water and frequency of wet mopping significantly reduced both p,p′-DDT and p,p′-DDE in mothers who reported ever having lived in a home sprayed with DDT, but not in mothers who reported never having lived in a home sprayed with DDT. For example, in mothers who reported ever having lived in a home sprayed with DDT, we estimated that if everyone wet mopped their floors at least seven times per week, they would have marginal geometric mean p,p′-DDT and p,p′-DDE serum concentrations 90.3 (95% CI: , ) and 306.8 (95% CI: , ) ng/g-lipid, respectively, lower than where everyone wet mopped their floors fewer than seven times per week. However, for mothers who reported never having lived in a home sprayed with DDT, we estimated that if everyone wet mopped their floors at least seven times per week, they would have marginal geometric mean p,p′-DDT and p,p′-DDE serum concentrations 7.1 (95% CI: , 12.4) and 29.7 (95% CI: , 36.8) ng/g-lipid, respectively, lower than where everyone wet mopped their floors fewer than seven times per week.
Discussion
Among women participating in the VHEMBE study in Limpopo, South Africa, p,p′ isomers of DDT/E were above the LOD in the majority of the serum samples with median (IQR) p,p′-DDT and p,p′-DDE concentrations of and , respectively. We found geographic variation in p,p′-DDT/E exposure in our study area. Using causal inference techniques, we estimated that mopping floors regularly would significantly reduce exposure to p,p′-DDT/E across the entire VHEMBE population, with the largest reduction in p,p′-DDT/E serum concentrations occurring in the population of mothers reporting ever having lived in a home sprayed with DDT.
Direct comparisons of p,p′-DDT/E serum concentrations in the VHEMBE cohort with previously reported blood concentrations in IRS areas was difficult due to different exposure classification metrics used across studies (Table S5). Despite this difficulty, we did find similarities in our results with the more recent blood concentrations reported in IRS communities. For example, p,p′-DDT/DDE concentrations from VHEMBE mothers reporting to have lived in a home sprayed with DDT during pregnancy were similar to concentrations among women in the SOWB who lived in a home “probably sprayed with DDT” () (p,p′-DDT medians: 736.9 vs. -lipid; p,p′-DDE medians: 2,129.0 vs. -lipid) (Whitworth et al. 2014). In addition, p,p′-DDT/E concentrations from VHEMBE mothers reporting that they had never lived in a home sprayed with DDT were similar to concentrations among delivering women in a “low-risk malaria area” () of KwaZulu-Natal, South Africa (p,p′-DDT medians: 33.5 vs. -lipid; p,p′-DDE medians: 155.8 vs. -lipid) (Channa et al. 2012). However, our p,p′-DDT/DDE concentrations were less congruent with older biomonitoring studies. For example, p,p′-DDT/E concentrations from VHEMBE mothers reporting to have lived in a home sprayed with DDT during pregnancy were substantially lower than concentrations from males living in DDT-sprayed homes collected between 2003–2005 reported in Aneck-Hahn et al. (2007) (p,p′-DDT medians: 736.9 vs. -lipid; p,p′-DDE medians: 2,129.0 vs. -lipid, respectively). Biomonitoring data do indicate decreased DDT/DDE blood concentrations by date of serum/plasma collection, potentially due to a recent reduction in the use of DDT for IRS in the Vhembe district.
In comparison with non-IRS communities in the U.S., VHEMBE mothers’ p,p′-DDT/DDE concentrations were higher than levels found in pregnant women participating in the NHANES (p,p′-DDT medians: 55.3 vs. ; p,p′-DDE medians: 242.2 vs. -lipid, respectively) (Center for Disease Control 2000, 2002, 2004). VHEMBE p,p′-DDT concentrations were typically higher than concentrations in pregnant women participating in the Center for the Health Assessment of Mother and Children of Salinas (CHAMACOS) study conducted in 2000 of pregnant women, who were mostly recent Mexican immigrants, in California (medians: 55.3 vs. -lipid, respectively), but the p,p′-DDE concentrations were typically lower than in results from the CHAMACOS study (medians: 242.2 vs. -lipid, respectively) (Bradman et al. 2007). It should be noted that many of the CHAMACOS mothers came from coastal areas of Mexico where DDT was used for malaria control until 2000 (Chanon et al. 2003). VHEMBE p,p′-DDT/E concentrations were typically lower than historical blood samples collected during the period of intensive DDT use in the U.S. (Bhatia et al. 2004; Jusko et al. 2012). However, VHEMBE mothers who reported that they lived in a home sprayed with DDT for IRS during pregnancy had p,p′-DDT/DDE serum concentrations that approached the historical concentrations during intensive DDT use in the U.S. (p,p′-DDT medians: 736.9 vs. ; p,p′-DDE medians: 2,129 vs. ). However, home spray history was self-reported and, without formal confirmation, we may have underestimated exposure in women from sprayed homes.
We found a clear geographic variation in p,p′-DDT and p,p′-DDE serum concentrations in the VHEMBE study area. Mothers living in the southern and western regions typically had higher serum concentrations than mothers living in the north and east. The geographic variation of p,p′-DDT and p,p′-DDE exposures are likely due to the topography of study area, as the northern and eastern regions are at a higher elevation where mosquitos are less likely to survive, thereby reducing the need for IRS.
Our results show that living in a home sprayed with DDT was associated with significantly higher DDT/DDE serum levels. Given this finding and previous findings of elevated DDT/DDE serum and plasma concentrations in those living in sprayed homes and villages (Aneck-Hahn et al. 2007; Bouwman et al. 2006; Channa et al. 2012; Herrera-Portugal et al. 2005; Manaca et al. 2011; Ortiz-Pérez et al. 2005; Sereda et al. 2009; Van Dyk et al. 2010; Whitworth et al. 2014), it is clear that individuals living in IRS sprayed homes are being exposed to higher levels of insecticides from the contamination of their immediate environment. Yet, the majority of mothers living in a home sprayed during their pregnancies were inside the home during IRS (80%) and did not move household items outside before IRS (58%), despite these precautions having been advocated by WHO (World Health Organization 2007). Our results indicate that spray workers and/or supervisors need to better promote insecticide safety protections advocated by WHO with the residents of IRS sprayed homes to limit their exposure.
Similar to associations found by Whitworth et al. (2014), we estimated that having water piped into homes reduced p,p′-DDT exposure, with the largest reduction in exposure occurring in women reporting ever having lived in a home sprayed with DDT in our causal inference analysis. It should be noted that homes with concrete-brick walls were more likely to have water piped into the homestead () than more traditional rondavel homes with earthen walls () and concrete-brick walls are more likely to be painted than earthen walls. DDT is not supposed to be sprayed on painted surfaces as the insecticide does not adhere to the walls; therefore, the association between water access and DDT exposure may be confounded by home type. To prevent this confounding “back-door” pathway, we accounted for both pregnancy home type and presence of a rondavel on the homestead in our causal models. We did not control for frequency of wet mopping or washing clothes when we targeted the impact of access to water on DDT/DDE serum levels because cleaning behaviors are along the causal pathway between intervention and outcome. Therefore, the impact of access to water on reducing DDT/DDE exposure encompasses the impact of cleaning frequency.
To our knowledge, this is the first study to report that frequency of cleaning may reduce DDT or DDE exposure. Previous studies on the impact of cleaning on insecticide exposure are scarce. In an intervention study of inner-city pregnant women (intervention , control ), mothers who received a professional home cleaning and education following application of the pesticide propoxur had no detectable plasma concentrations of cis-permethrin, trans-permethrin, and 2-isopropoxyphenol. and detection frequencies were between 11.8–29.4% among controls (Williams et al. 2006). However, a small cleaning intervention study () did not find that wet mopping of linoleum floors with soap was effective at removing organophosphate pesticide residues (McCauley et al. 2006). If our finding that frequency of wet mopping reduced DDT/DDE exposure are reproduced, the promotion of household cleaning through education may be a viable intervention in IRS communities to reduce insecticide exposure.
As with all public health policies, health officials should ensure that they are doing no harm with the exposure-reduction interventions proposed. For example, a public health policy that reduces the efficacy of IRS for malaria control would not be an appropriate intervention. Through this research, we have found that increasing access to water and wet mopping of floors may be potential interventions, and we do not believe they would have negative consequences. Although increasing access to water would be a financial burden on governments, residents would receive the benefits of increased sanitation, reducing the time burden of collecting water, and potentially reducing exposure to DDT from IRS. Further, we believe that increased wet mopping would not have negative consequences and would not reduce the efficacy of IRS. Floors are not sprayed during IRS for malaria control because mosquitoes rest on walls after taking a blood meal. In the WHO IRS spray manuals, the authors advise residents to sweep or mop after IRS, indicating that WHO believes the presence of insecticide residues on floors to be unattended and not needed for malaria protection (World Health Organization 2007). In addition, we believe reducing DDT/DDE exposure in IRS communities by any amount to be advantageous, given that the International Agency for Research on Cancer classifies DDT as “probably carcinogenic to humans” (Loomis et al. 2015) and that the toxicity of carcinogens is generally viewed as being “non-threshold,” meaning that any level of exposure may pose a risk (Albert et al. 1977).
A key strength of this research study is the use of a causal inference analysis to test “intervenable” characteristics to reduce exposure to DDT/DDE. We designed our statistical analysis to be able to target potential interventions to reduce insecticide exposure to residents of homes sprayed for malaria control. To our knowledge, this study is the first to apply a causal inference methodology to test for interventions to reduce environmental xenobiotic exposures, and we advocate the continued use of this methodology to better inform public health policies. In addition, the use of TMLE using data-adaptive machine learning algorithms allowed for a targeted approach, where the research question defines the statistical analysis, to understand the effect of interventions on DDT/DDE body burden, without a priori specifying a model’s input or making assumptions about the form of the respective relationships among exposure, outcome, and covariates. Further, this study is the largest biomonitoring effort in a population with close geographic proximity to IRS to date. We collected a diverse set of demographic/exposure information, and we were able to observe home characteristics directly during the one-week visits. We also were able to limit the amount of missing data and obtained high participant retainment between the delivery and one-week visit.
A limitation of this study was the reliance on questionnaire data to ascertain if the mother’s home or village had ever been sprayed and sprayed during her pregnancy for malaria control. Although spray operators are supposed to fill out “spray cards” at each home, few participants had these cards. Even if current home spray cards were filled out, due to DDT and DDE’s long biological half-life (ATSDR 2002), knowledge of IRS spray history for all homes a mother had lived in (not just the spray card from her current home) is necessary for an accurate exposure assessment and we may have underestimated exposure among those living in sprayed homes. Despite this limitation, the housing/village IRS exposure variables were associated with DDT/E serum levels, giving strength to the assumption that women did accurately report their exposure classifications. However, better data on IRS coverage in communities is essential for controlling malaria more efficiently and applying insecticides more judiciously. A potential future direction is the use of cell-phone technology to better track coverage of IRS (Eskenazi et al. 2014). In addition, our study results are derived from a population of Limpopo women enrolled with our eligibility criteria; therefore, our results may not be translatable to all populations protected using IRS for malaria control.
Conclusion
Findings of this study suggest that increased access to water and frequency of wet mopping in homes ever sprayed with DDT may mitigate p,p′-DDT and p,p′-DDE exposure in IRS communities. Given the potential health effects of exposure to IRS insecticides, further investigations of exposure interventions are warranted to effectively and safely eliminate malaria.
Supplemental Material
Acknowledgement
Funding from the National Institute of Environmental Health Sciences, Award ID: 1R01ES020360-01.
References
- Albert RE, Train RE, Anderson E. 1977. Rationale developed by the Environmental Protection Agency for the assessment of carcinogenic risks. J Natl Cancer Inst 58(5):1537–1541, PMID: 853532, 10.1093/jnci/58.5.1537. [DOI] [PubMed] [Google Scholar]
- Aneck-Hahn NH, Schulenburg GW, Bornman MS, Farias P, de Jager C. 2007. Impaired semen quality associated with environmental DDT exposure in young men living in a malaria area in the Limpopo Province, South Africa. J Androl 28(3):423–434, PMID: 17192596, 10.2164/jandrol.106.001701. [DOI] [PubMed] [Google Scholar]
- ATSDR. 2002. Toxicological Profile for DDT, DDE, and DDD. Atlanta, GA:Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/. [PubMed]
- Barr JR, Maggio VL, Barr DB, Turner WE, Sjödin A, Sandau CD, et al. 2003. New high-resolution mass spectrometric approach for the measurement of polychlorinated biphenyls and organochlorine pesticides in human serum. J Chromatogr B Analyt Technol Biomed Life Sci 794(1):137–148, 10.1016/S1570-0232(03)00451-3. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Shiau R, Petreas M, Weintraub JM, Farhang L, Eskenazi B. 2004. Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ Health Perspect 113(2):220–224, PMID: 15687061, 10.1289/ehp.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman H, Sereda B, Meinhardt HM. 2006. Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa. Environ Pollut 144(3):902–917, PMID: 16564119, 10.1016/j.envpol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Bradman AS, Schwartz JM, Fenster L, Barr DB, Holland NT, Eskenazi B. 2007. Factors predicting organochlorine pesticide levels in pregnant Latina women living in a United States agricultural area. J Expos Sci Environ Epidemiol 17(4):388–399, PMID: 17033681, 10.1038/sj.jes.7500525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2000. 1999–2000 National Health and Nutrition Examination Survey (NHANES), Demographics and Dioxins Data Files. Atlanta, GA:Centers for Disease Control and Prevention; http://wwwn.cdc.gov/nchs/nhanes/search/nhanes99_00.aspx. [Google Scholar]
- Centers for Disease Control and Prevention. 2002. 2001–2002 National Health and Nutrition Examination Survey (NHANES), Demographics and Dioxins Data Files. Atlanta, GA:Centers for Disease Control and Prevention; http://wwwn.cdc.gov/nchs/nhanes/search/nhanes01_02.aspx. [Google Scholar]
- Centers for Disease Control and Prevention. 2004. 2003–2004 National Health and Nutrition Examination Survey (NHANES), Demographics and Dioxins Data Files. Atlanta, GA:Centers for Disease Control and Prevention; http://wwwn.cdc.gov/nchs/nhanes/search/nhanes03_04.aspx. [Google Scholar]
- Channa K, Röllin HB, Nøst TH, Odland JØ, Sandanger TM. 2012. Prenatal exposure to DDT in malaria endemic region following indoor residual spraying and in non-malaria coastal regions of South Africa. Sci Total Environ 429:183–190, PMID: 22578843, 10.1016/j.scitotenv.2012.03.073. [DOI] [PubMed] [Google Scholar]
- Chanon KE, Mendez-Galvan JF, Galindo-Jaramillo JM, Olguin-Bernal H, Borja-Aburto VH. 2003. Cooperative actions to achieve malaria control without the use of DDT. Int J Hyg Environ Health 206(4-5):387–394, PMID: 12971694, 10.1078/1438-4639-00235. [DOI] [PubMed] [Google Scholar]
- Efron B. 1979. Bootstrap methods: Another look at the jackknife. Ann Statist 7(1):1–26, 10.1214/aos/1176344552. [DOI] [Google Scholar]
- Eskenazi B, Quirós-Alcalá L, Lipsitt JM, Wu LD, Kruger P, Ntimbane T, et al. 2014. mSpray: A mobile phone technology to improve malaria control efforts and monitor human exposure to malaria control pesticides in Limpopo, South Africa. Environ Int 68:219–226, PMID: 24769412, 10.1016/j.envint.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco JP, Achee NL, Chareonviriyaphap T, Suwonkerd W, Chauhan K, Sardelis MR, et al. 2007. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS One 2:e716, PMID: 17684562, 10.1371/journal.pone.0000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Portugal C, Ochoa H, Franco-Sánchez G, Yáñez L, Díaz-Barriga F. 2005. Environmental pathways of exposure to DDT for children living in a malarious area of Chiapas, Mexico. Environ Res 99(2):158–163, PMID: 16194665, 10.1016/j.envres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Jean WH, Helms BP. 1983. Geometric mean approximations. J Fin Quant Anal 18(3):287–293, 10.2307/2330720. [DOI] [Google Scholar]
- Jusko TA, Klebanoff MA, Brock JW, Longnecker MP. 2012. In-utero exposure to dichlorodiphenyltrichloroethane and cognitive development among infants and school-aged children. Epidemiology 23(5):689–698, PMID: 22766752, 10.1097/EDE.0b013e31825fb61d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Fedak K, Kramer R. 2012. Reduction of malaria prevalence by indoor residual spraying: A meta-regression analysis. Am J Trop Med Hyg 87(1):117–124, PMID: 22764301, 10.4269/ajtmh.2012.11-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis D, Guyton K, Grosse Y, El Ghissasi F, Bouvard V, Benbrahim-Tallaa L, et al. 2015. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol 16(8):891–892, PMID: 26111929, 10.1016/S1470-2045(15)00081-9. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112(17):1691–1696, PMID: 15579415, 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabaso MLH, Sharp B, Lengeler C. 2004. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health 9(8):846–856, PMID: 15303988, 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- MacIntyre UE, Venter CS, Vorster HH. 2001a. A culture-sensitive quantitative food frequency questionnaire used in an African population: 1. Development and reproducibility. Public Health Nutr 4(1):53–62, PMID: 11315681, 10.1079/PHN200040. [DOI] [PubMed] [Google Scholar]
- MacIntyre UE, Venter CS, Vorster HH. 2001b. A culture-sensitive quantitative food frequency questionnaire used in an African population: 2. Relative validation by 7-day weighted records and biomarkers. Public Health Nutr 4(1):63–71, PMID: 11315682, 10.1079/PHN200041. [DOI] [PubMed] [Google Scholar]
- MacIntyre UE, Venter CS, Vorster HH, Steyn HS. 2001c. A combination of statistical methods for the analysis of the relative validation data of the quantitative food frequency questionnaire used in the THUSA study. Transition, health and urbanisation in South Africa. Public Health Nutr 4(1):45–51, PMID: 11315680, 10.1079/PHN200039. [DOI] [PubMed] [Google Scholar]
- Manaca MN, Grimalt JO, Sunyer J, Mandomando I, Gonzalez R, Sacarlal J, et al. 2011. Concentration of DDT compounds in breast milk from African women (Manhiça, Mozambique) at the early stages of domestic indoor spraying with this insecticide. Chemosphere 85(3):307–314. PMID: 21764104, 10.1016/j.chemosphere.2011.06.015. [DOI] [PubMed] [Google Scholar]
- McCauley LA, Travers R, Lasarev M, Muniz J, Nailon R. 2006. Effectiveness of cleaning practices in removing pesticides from home environments. J Agromedicine 11(2):81–88, PMID: 17135145, 10.1300/J096v11n02_11. [DOI] [PubMed] [Google Scholar]
- Nutritional Intervention Research Unit and Biomedical Research Division. 2011. FoodFinder 3. http://foodfinder.mrc.ac.za/home.htm.
- Ortiz-Pérez MD, Torres-Dosal A, Batres LE, López-Guzmán OD, Grimaldo M, Carranza C,. et al. 2005. Environmental health assessment of deltamethrin in a malarious area of Mexico: Environmental persistence, toxicokinetics, and genotoxicity in exposed children. Environ Health Perspect 113(6):782–786, PMID: 15929904, 10.1289/ehp.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl J. 2000. Causality: Models, Reasoning and Inference. Cambridge, UK: Cambridge Univ Press. [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr., Henderson LO, Needham LL. 1989. Chlorinated hydrocarbon levels in human serum: Effects of fasting and feeding. Arch Environ Contam Toxicol 18(4):495–500, PMID: 2505694, 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Ritter SJ, Jewell NP, Hubbard AE. 2014. R Package multiPIM: A causal inference approach to variable importance analysis. J Stat Softw 57(8):1–29, 10.18637/jss.v057.i08.25400517 [DOI] [Google Scholar]
- Rose S, van der Laan MJ. 2011. Why TMLE? In: Targeted Learning. New York: Springer, 101–118. [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. 2009. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 20(4):488, PMID: 19525685, 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereda B, Bouwman H, Kylin H. 2009. Comparing water, bovine milk, and indoor residual spraying as possible sources of DDT and pyrethroid residues in breast milk. J Toxicol Environ Health Part A 72(13):842–851, PMID: 19557612, 10.1080/15287390902800447. [DOI] [PubMed] [Google Scholar]
- Statistics South Africa. 2014. Poverty Trends in South Africa: An Examination of Absolute Poverty between 2006 and 2011. Pretoria, South Africa:Statistics South Africa. [Google Scholar]
- Textor J, Hardt J, Knüppel S. 2011. DAGitty: A graphical tool for analyzing causal diagrams. Epidemiology 22(5):745, PMID: 21811114, 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- van der Laan MJ. 2006. Statistical inference for variable importance. Int J Biostat 2(1):2, 10.2202/1557-4679.1008. [DOI] [Google Scholar]
- van der Laan MJ, Rubin D. 2006. Targeted maximum likelihood learning. Int J Biostat 2(1):11, 10.2202/1557-4679.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan MJ, Polley EC, Hubbard AE. 2007. Super Learner. Stat Appl Genet Mol Biol 6(1):25, PMID: 17910531, 10.2202/1544-6115.1309. [DOI] [PubMed] [Google Scholar]
- Van Dyk JC, Bouwman H, Barnhoorn IEJ, Bornman MS. 2010. DDT contamination from indoor residual spraying for malaria control. Sci Total Environ 408(13):2745–2752, PMID: 20381127, 10.1016/j.scitotenv.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Waliszewski SM, Aguirre AA, Infanzon RM, Siliceo J. 2000. Carry-over of persistent organochlorine pesticides through placenta to fetus. Salud Publica Mex 42(5):384–390, PMID: 11125622, 10.1590/S0036-36342000000500003. [DOI] [PubMed] [Google Scholar]
- Whitworth KW, Bornman RM, Archer JI, Kudumu MO, Travlos GS, Wilson RE, et al. 2014. Predictors of plasma DDT and DDE concentrations among women exposed to indoor residual spraying for malaria control in the South African Study of Women and Babies (SOWB). Environ Health Perspect 122(7):545–552, PMID: 24577839, 10.1289/ehp.1307025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MK, Barr DB, Camann DE, Cruz LA, Carlton EJ, Borjas M, et al. 2006. An intervention to reduce residential insecticide exposure during pregnancy among an inner-city cohort. Environ Health Perspect 114(11):1684–1689, PMID: 17107853, 10.1289/ehp.9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2007. Manual for Indoor Residual Spraying: Application of Residual Sprays for Vector Control. http://www.pmi.gov/docs/default-source/default-document-library/implementing-partner-reports/irs_taskorder1_finalreport.pdf?sfvrsn=4.
- World Health Organization. 2014a. WHO Recommended Insecticides for Indoor Residual Spraying against Malaria Vectors. Updated Nov. 17th, 2014.
- World Health Organization. 2014b. World Malaria Report. Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2014/en/ [accessed July 2014]. [Google Scholar]
- World Health Organization. 2015. World Malaria Report. Geneva, Switzerland: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ [accessed July 2014]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.