Abstract
Background
Most current prophylactic vaccines confer protection primarily through humoral immunity. Indeed, aluminum salts which have been widely used as adjuvants in vaccines primarily enhance Th2-driven antibody responses. Therefore, new vaccines formulation is moving toward a careful selection of adjuvants that also elicit significant Th1 or Tc1 responses. Several TLR agonists have been tested as potential new adjuvants in clinical and preclinical studies with some efficacy. These studies suggest that combining more than one of TLR ligands enhances the magnitude of immune responses to cancer and infectious disease.
Objectives
In order to evaluate the synergistic effect of TLR agonists for effective induction of cellular immunity, we investigated the effects of single and/or combined TLR agonists on monocyte-derived DC maturation, DC-NK crosstalk and ultimately naïve T cells polarization into effector T cells.
Results
Among the adjuvants tested, we found that TLR3, TLR4, TLR7/8 and TLR8 agonists were the most effective adjuvants to increase the expression levels of antigen-presenting, co-stimulatory molecules and production of cytokines by maturing DCs. When combined, TLR3+8 and TLR4+8 synergistically optimized DC maturation and IFN-γ secretion from NK cells co-cultured with DCs. Interestingly, co-culture of DC-NK-T treated with aluminum salt produced the highest percentage of effector memory CFSE−CCR7− Th1 cells whereas TLR3+8 and TLR4+8 treated co-cultures produced the highest percentage of effector memory CFSE−CCR7− Tc1 cells producing IFN-γ. Finally, while both TLR3+8 or TLR4+8 treated co-cultures generated similar frequency of Th1 and Tc1 effector cells, the effector cells from the latter co-culture produced quantitatively more IFN-γ in the supernatant.
Conclusion
Our data indicate that if in need of an enhanced DC-NK mediated cellular immunity one may select TLR agonists with defined synergistic effects.
Keywords: Dendritic cells, NK cells, Th1, Tc1, TLR agonists, DC-NK crosstalk
1. Introduction
Although there has been numerous research on adjuvants for use in vaccines over the last decade, very few adjuvants such as aluminum hydroxide, aluminum phosphate, and squalene-based oil-in-water emulsions have been licensed for human vaccines [1]. While more effective in inducing humoral immunity, none of the currently approved adjuvants uniformly or sufficiently enhance cellular immunity that is essential for the elimination of certain microorganisms, particularly intracellular pathogens and tumor cells [2]. Toll-like receptors (TLRs) are pathogen associated molecular pattern (PAMP) recognition receptors that are an important link between innate and adaptive immunity [3]. They are expressed on most innate immune cells including dendritic cells (DC), nature killer cells (NK), monocytes and granulocytes [4]. PAMPs or TLR agonists mediate the interaction between DCs and NK cells, which is a critical step in initiating an adaptive immunity. Indeed, following TLR engagement, DCs migrate from peripheral tissues to lymphoid organs while up-regulating major histocompatibility complex and costimulatory molecules, and acquiring the unique capacity to prime naive T cells [5]. In draining lymph nodes, DCs determine the character of the ensuing immune response by secreting cytokines (e.g., IL-12) that drive the development of pathogen specific naïve T cells into helper T cell type 1 (Th1) and cytotoxic T cell type 1 (Tc1) secreting IFN-γ [6]. DCs also potentiate their own efficiency by interacting with NK cells recruited to the lymphoid tissues in response to PAMPs. IL-12 released by mature DCs can activate NK cells. In turn, NK cells that are activated by IL-12 and PAMPs through their own TLRs release IFN-γ necessary for enhancing stable IL-12 production by DCs and maintaining Th1 cell polarization [7]. Therefore, the ability to promote DC-NK crosstalk has been considered as a criterion for selection of Th1 adjuvants.
Two classes of TLRs can be defined based on their cellular localization: TLRs 1, 2, 4–6 are expressed on the cell surface and recognize pathogenic components, while TLRs 3, 7, 8 and 9 are expressed in intracellular endosome/lysosome membranes and are nucleic acid sensors [8]. In most instances signaling through TLRs favors the development of a Th1 response, which has triggered interest in exploiting TLR agonists as potential adjuvants for prophylactic and therapeutic vaccines [1]. Indeed, number of published clinical trials utilizing TLRs 2, 3, 4, 7/8 and 9 concluded that TLR ligands are safe, well-tolerated, and effective vaccine adjuvants [1, 4, 9–11].
Both monocyte-derived DCs (MoDCs) and human blood myeloid DCs express TLR1-8 and not TLR9 which is only detectable in plasmacytoid DCs [12]. Due to low frequency of circulating DCs in human blood, ex vivo-cultured MoDCs have been used predominantly to evaluate the efficacy of a single TLR agonist to provoke maturation, antigen presentation, cytokine production, and subsequent Th1 polarization of DCs [13–21]. Because pathogens express more than one PAMP, some of these studies have also investigated the benefit of combining TLR3 (Poly IC), TLR4 (LPS), TLR7/8 (R848) agonists as an approach to generate strong T cell immunity that is required for elimination of intracellular pathogens and cancer cells. DCs treated by TLR4 plus TLR7/8 were induced to secrete much higher IL-12 and possess Th1 polarization than were DCs treated by single agonists [14, 17–20]. The cocktail containing TLR3 and TLR7/8 also induced much higher secretion of IL-12 by DCs, but it did not further enhance their Th1 promoting capacity when compared with TLR4 or TLR7/8 alone [16]. In another study, DCs generated with mixtures of inflammatory cytokines, TLR3, and TLR8 had superior capacity to activate NK cells and prime Th1 polarized cells when compared with DCs matured without additional TLR agonists or with only the TLR3 agonist [21]. We have expanded on these published studies and evaluated the influence of single and combined TLR agonists including less toxic derivative of TLR4 agonist, i.e., MPLA and three TLR7/8 agonists, i.e., TLR7 (CL246), TLR7/8 (R848), and TLR8 (CL075) on human DC, DC-NK crosstalk and DC-NK-T cells interactions. Our data suggest that among the TLR agonists tested, TLR8 synergizes with TLR4 or TLR3 to program DCs for optimal DC-NK mediated polarization of IFN-γ producing CD4 (Th1) and in particular CD8 (Tc1) effector T cells.
2. Materials and methods
2.1. Media and reagents
Complete culture media (CM) contained RPMI 1640, 1% L-glutamine, 1% penicillin/streptomycin, 50 μM 2β-Mercaptoethanol, 1% sodium-pyruvate, 1% non-essential amino acids and heat-inactivated 10% Fetal Calf Serum (FCS). Recombinant human Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF), Interleukin (IL)-4, IL-12 and IL-2 were purchased from Peprotech (Rocky Hill, NJ). TLR agonists were purchased from Invivogen (San Diego, CA). Media for staining cells contained PBS 1X without Ca2+/Mg2+, heat-inactivated 2% FCS and 2 mM EDTA.
2.2. Generation and immunophenotyping of DCs
Immature monocyte-derived DCs were generated from peripheral blood adhering monocytes cultured in CM with GM-CSF (100 ng/mL) and IL-4 (10 ng/mL). Cultures were fed every 2 days with CM containing cytokines. Day 6 immature DCs (2.5×105/ml) were suspended in CM and activated for 48hrs with aluminum hydroxide gel 2% (Alum, 2μl/ml) or TLR agonists TLR2 (Pam3CSK4, 1μg/ml), TLR3 (Polyinosinic I:C, 5μg/ml), TLR4 (Monophosphoryl Lipid A, 2.5μg/ml), TLR5 (Flagellin, 1μg/ml), TLR7 (Adenine analog CL264, 2.5μg/ml), TLR7/8 (Imidazoquinoline R848, 2.5μg/ml), TLR8 (Thiazoquinoline CL075, 2.5μg/ml), and TLR9 (Type C CpG oligonucleotide ODN 2395, 5μg/ml). Immature and mature DCs were stained with fluorochrome-labeled monoclonal Antibodies (mAbs) CD1a (HI149), CD14 (61D3), HLA-DR (LN3), CD80 (L307.4), CD83 (HB15a), CD86 (IT2.2) and CD40 (5C3) (BD Biosciences, San Jose, CA and eBiosciences, San Diego, CA) and analyzed by multi-color Flow Cytometry. The supernatants of mature DC cultures were analyzed by ELISA for production of cytokines, TNF-α, IL-1β, IL-10 and IL-12 (eBioscience and BD Bioscience, San Diego, CA). The supernatants were also used as DC-conditioned media.
2.3. NK cell isolation
NK cells were isolated from peripheral blood mononuclear cells (PBMCs) using the EasySep Human NK cell Enrichment Kit (StemCell Technologies, Vancouver, Canada). The purity of the enriched NK cell population was >90% based upon prevalence of CD56+CD3- phenotypes.
2.4. DC-NK crosstalk
Immature DCs and NK cells (1×105 DC: 2×105 NK/well) were exposed to Alum or TLR agonists. NK cells alone were also stimulated with indicated TLR agonists in the presence of IL-12 (5ng/ml) or DC-conditioned media. Supernatants collected from day 2 DC-NK co-cultures and NK cultures were analyzed by ELISA for IL-12 and IFN-γ production by DCs and NK cells, respectively. NK cells were also stained with corresponding fluorochrome-labeled mAbs CD3 (UCHT1), CD56 (MEM188), and CD69 (L78) and analyzed by Flow Cytometry for activation status of NK cells.
2.5. DC-NK-T cells co-culture
Syngeneic immature DCs and NK cells (1×105 DC: 2×105 NK/well) exposed to Alum or TLR agonists for 8hrs were subsequently co-cultured in 48-well plates (Falcon, Franklin Lakes, NJ) at a 1:10 ratio with CFSE labeled allogeneic naïve T cells isolated from PBMCs using T cell isolation kit (StemCell Technologies, Vancouver, Canada). The purity of the enriched T cells was >95% based upon prevalence of CD3+CD56- phenotypes. On day 5, the proliferating cells were transferred into new plates and rested in IL-2-containing medium (5ng/ml) up to 10 days. The cells were subsequently collected and stained with CD4 (L200), CD8 (SK1), and CCR7 (3D12) (primary co-cultures). The remaining T cells were transferred to plates pre-coated with 10μg/ml mAbs CD3 (UCHT1) and 2μg/ml soluble CD28 (CD28.2) (BD Biosciences, San Diego, CA) for 72hrs. The T cells were then stimulated for 4–6hrs with leukocyte activation cocktail (BD Biosciences) containing Brefeldin A before staining with CD4 (L200), CD8 (SK1), CCR7 (3D12), and intracellular IFN-γ (4S.B3) (BD Biosciences, San Diego, CA) (secondary co-cultures). The frequency and amount of IFN-γ were further analyzed using Flow Cytometry and ELISA.
2.6. Statistical analysis
Statistical significance of differences between Alum and TLR agonists was calculated using unpaired Mann-Whitney test. A p value <0.05 was considered statistically significant and shown with an asterisk (*). Analysis was performed using a Prism program (GraphPad, San Diego, CA). Results are expressed as mean ± SEM. Synergistic effect is considered as ≥ 3-fold increase in the sum of individual TLR agonist effects on the expression or production of indicated parameters.
3. Results
3.1. Maturation and cytokines production of human MoDCs in response to single TLR agonists
In response to maturational stimuli, DCs not only increase the expression of antigenpresenting and co-stimulatory molecules, but also produce pro-inflammatory cytokines [22, 23]. Maturation of DCs is critical for optimal activation, proliferation and final differentiation of naïve T cells to effector memory T cells [24]. Thus, we first evaluated the changes in expression of antigen-presenting and co-stimulatory molecules as well as the production of pro-inflammatory cytokines by maturing DCs in response to single TLR agonists, TLR2 (Pam3CSK4), TLR3 (Poly I:C), TLR4 (MPLA), TLR5 (Flagellin), TLR7/8 (R848), TLR8 (CL075), and TLR9 (CpG). Our data revealed that all TLR agonists moderately and variably increased the expression level of antigen-presenting molecule, HLA-DR, on maturing DCs when compared with Alum (Figure 1). Interestingly, further assessment of treated DCs showed that TLR5, TLR7 and TLR9 had minimal effect, while TLR2, TLR3 and to a greater extent TLR4, TLR7/8, and TLR8 agonists significantly increased the expression levels of maturational marker CD83 and costimulatory molecules CD40, CD80 and CD86 involved in T-cell priming (Figure 1). Maturing DCs also responded to Alum and the TLR agonists by producing different levels of proinflammatory and anti-inflammatory cytokines. Alum, TLR5, and TLR7 treated DCs produced minimal amounts of TNF-α, IL-1β, IL-10, and IL-12, a key cytokine for Th1 polarization. When compared with Alum, DCs treated with TLR2, TLR3, TLR4, TLR7/8, TLR8, and TLR9 secreted significantly more TNF-α; DCs treated with TLR8 secreted more IL-1β; DCs treated with TLR4, TLR7/8, TLR8 secreted more IL-10; and DCs treated with TLR3, TLR4, TLR7/8 and TLR8 secreted more IL-12 (Figure 2). The data suggests that similar to Alum, TLR2, TLR5, TLR7, and TLR9 agonists were the least effective adjuvants to increase the expression levels of antigen-presenting, co-stimulatory molecules and production of cytokines by maturing DCs (Figures 1 & 2). Since TLR3, TLR4, TLR7/8 and TLR8 showed to be overall the most effective agonists to induce DC maturation (Figures 1 & 2), we next tested whether various combinations of these selected TLR agonists could act additively or synergistically to further optimize the DC maturation.
Fig 1.
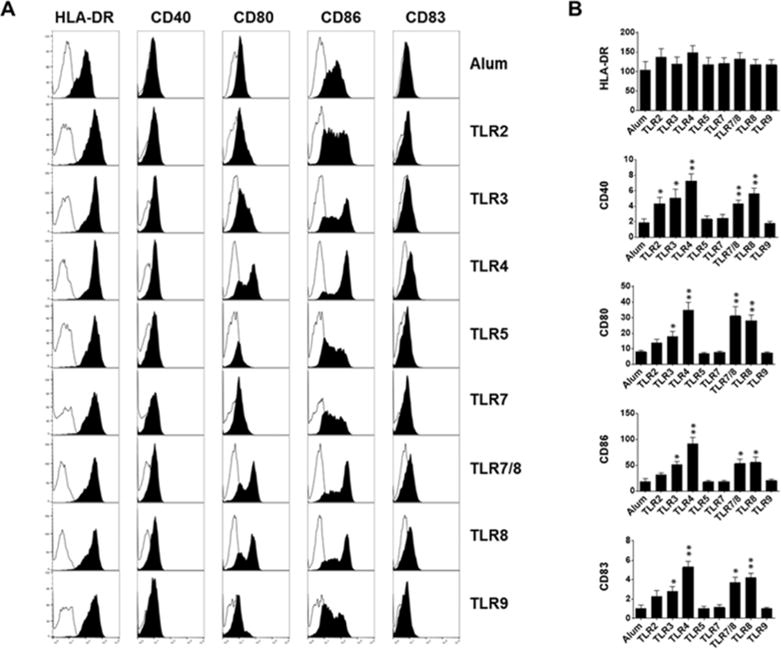
Immature DCs exposure to aluminum salt or indicated TLR agonists. A) Plots display overlaid histograms of indicated cell surface maturational markers (filled black) and control (filled white) on DCs after 48hr stimulation. One representative flow cytometry data is shown. B) Bar graphs show the geometric mean fluorescence intensity (± SEM) of indicated surface makers expressed on DCs (n=5–12, n denotes number of individual donor-derived MoDCs). * p<0.05, ** p<0.005, relative to Alum.
Fig 2.
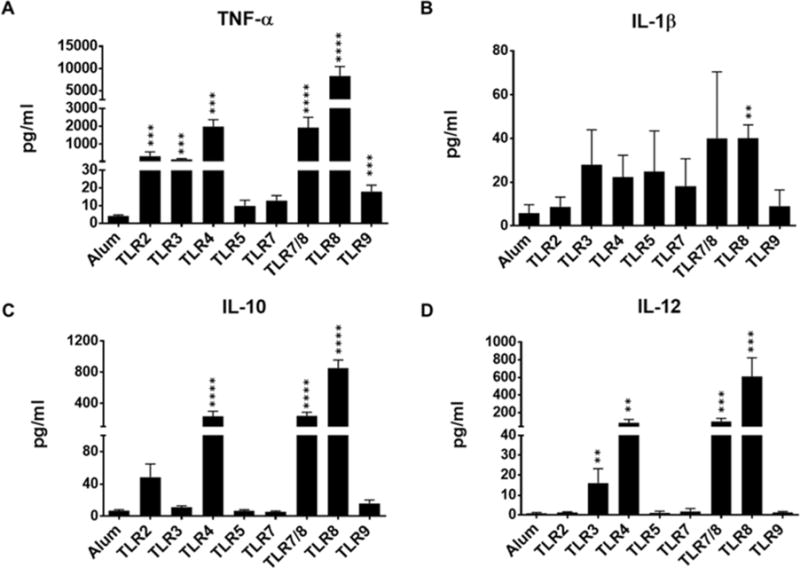
Immature DCs exposure to aluminum salt or indicated TLR agonists. A–D) Bar graphs show the amount of indicated cytokines released by DCs in the supernatants after 48hr stimulation. Data are expressed as mean ± SEM (n=4-17, n denotes number of individual donors). * p<0.05, ** p<0.005, *** p<0.001, **** p<0.0001, relative to Alum.
3.2. Maturation and cytokines production of human MoDCs in response to combined TLR agonists
Live, attenuated vaccines are more effective than subunit vaccines [1] due to the fact that upon exposure to the pathogens the immune cells recognize multiple PAMPs through their TLRs and each TLR is coupled with a specific signal transduction pathway [25, 26]. Therefore, we next evaluated the combined effects of TLR3, TLR4, TLR7/8, and TLR8 agonists that showed to be most effective in inducing DC expression of co-stimulatory molecules and production of inflammatory cytokines (Figures 1 & 2). We found no synergistic effects on DC expression of HLA-DR, CD40, CD80, CD86 and CD83 with any indicated TLR combinations (Figure 3). In addition, some combinations, particularly TLR7/8 and TLR8 demonstrated no significant effects on DC expression of costimulatory and maturation markers when compared with Alum (Figure 3). We also assessed the amount of cytokines (TNF-α, IL-1β, IL-10 and IL-12) secreted by DCs treated with the combined TLR agonists. Interestingly our data shows that in contrast to having almost no direct impact on DC maturational markers (Figure 3), all TLR combinations influenced the amount of the cytokines produced by treated DCs (Figure 4). TLR3+4 combination altered additively TNF-α, IL-12, and IL-10 and less-than-additive IL-1β production. TLR3+7/8 combination altered synergistically TNF-α, IL-10, IL-12 and less-than-additive IL-1β production. TLR3+8, TLR4+7/8, and TLR4+8 combinations synergistically increased all cytokines production. Finally, TLR7/8+8 combination altered less-than-additive TNF-α, IL-10, IL-1β, and IL-12 productions (Figure 4). Overall, our data show that TLR3+7/8, TLR3+8, TLR4+7/8 combinations had less synergistic effects on DC cytokines production, whereas the TLR4+8 combination was able to enhance the production of TNF-α (7-fold), IL-1β (52-fold), IL-10 (6-fold), and IL-12 (45-fold) when compared with the highest single producer, TLR8 agonist (Figures 2 & 4). We next examined whether TLR3+8 or TLR4+8, the highest producers of IL-12, could act in synergy and effectively program DCs for further DC-NK crosstalk and Th1 polarization.
Fig 3.
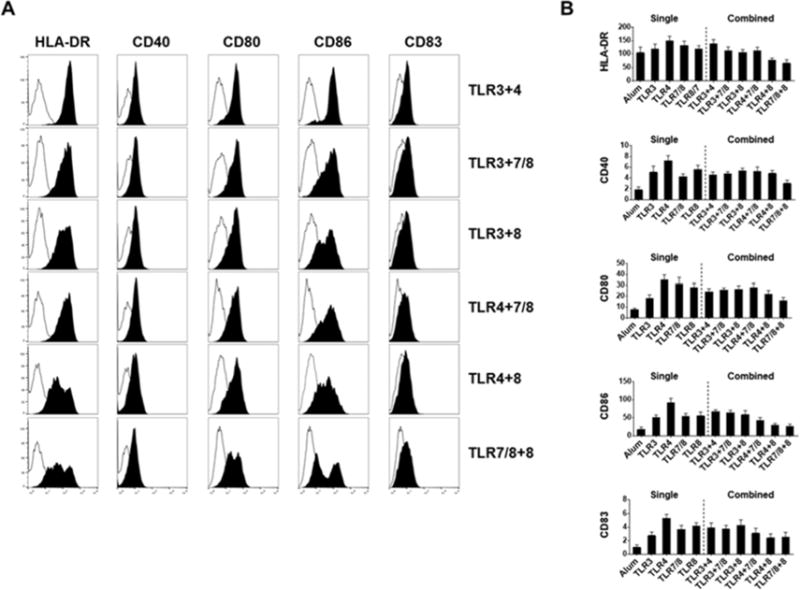
Immature DCs exposure to aluminum salt or indicated combination of TLR agonists. A) Plots display overlaid histograms of indicated cell surface maturational markers (filled black) and control (filled white) on DCs after 48hr stimulation. One representative flow cytometry data is shown. B) Bar graphs show the geometric mean fluorescence intensity (± SEM) of indicated surface makers expressed on DCs (n=3-9, n denotes number of individual donors). * p<0.05, ** p<0.005, relative to Alum.
Fig 4.
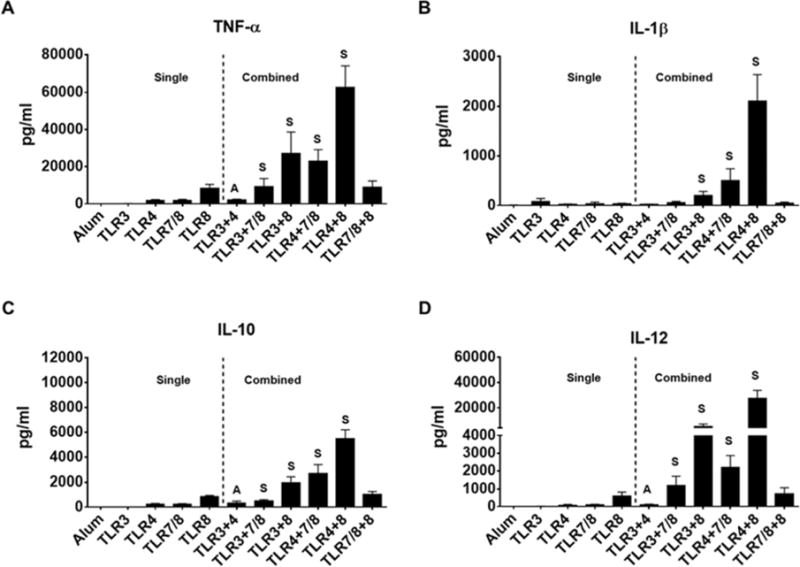
Immature DCs exposure to aluminum salt or indicated combination of TLR agonists. A–D) Bar graphs show the amount of indicated cytokines released by DCs in the supernatants after 48hr stimulation. Data are expressed as mean ± SEM (n=4-9, n denotes number of individual donors). * p<0.05, ** p<0.005, *** p<0.001, relative to Alum. S and A indicate synergistic and additive effects, respectively.
3.3. Human MoDC-NK crosstalk in response to selected TLR agonists
DC and NK interaction during the innate immune responses to pathogen is thought to provide an early source of IFN-γ necessary for enhancing the generation of effector memory T cells [27]. In order to measure the extent to which selected TLR agonists promote DC mediated NK cells activation, we cultured immature DCs with isolated NK cells and exposed them to indicated TLR agonists. When compared with Alum, single TLR agonists and their combinations significantly increased the percentage of the NK cells expressing the activation marker CD69 (Figure 5A, B). In addition, they promoted the production of IFN-γ with the exception of TLR3 agonist (Figure 5C). Interestingly, the optimal activation of NK cells was also dependent on direct DC-NK crosstalk since the sole addition of DC cytokine, IL-12, or DC-conditioned media moderately increased the expression level of CD69 and production of IFN-γ by NK cells exposed to the same TLR agonists (Figure 5). Finally, TLR3+8 and TLR4+8 combinations induced the highest amounts of IL-12 and acted additively to promote the maximal production of Th1 polarizing cytokine, IFN-γ, during DC-NK co-culture (Figure 5D). We next compared how effectively TLR3+8 or TLR4+8 prime the development of IFN-γ producing effector T cells.
Fig 5.
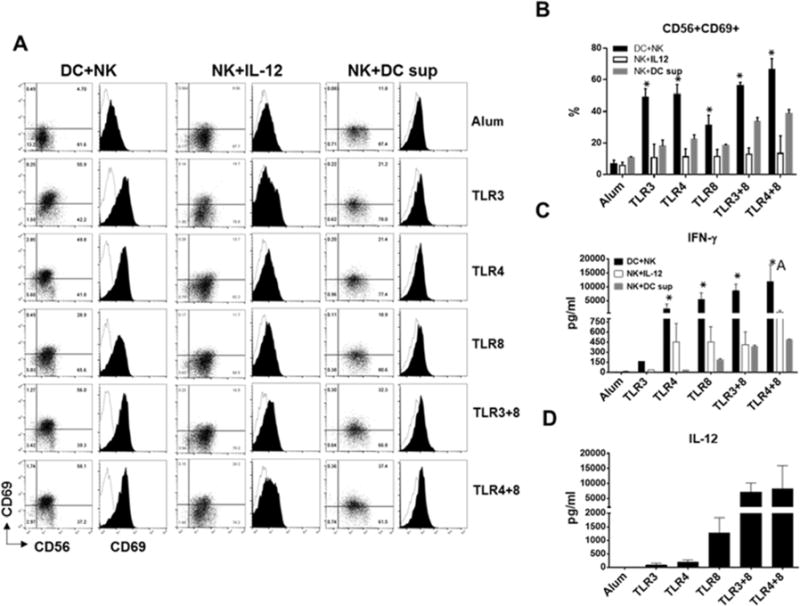
DCs and NK cells exposure to aluminum salt or indicated TLR agonists. A) Dot plots and overlaid histograms display percentage of CD56+CD69+ NK cells and expression of activation marker CD69 (filled black) on pre-gated CD56+CD3- NK cells after 48hr culture with DCs, IL-12 or DC-conditioned media. One representative flow cytometry data is shown. B) Bar graphs shows the percentage of CD56+CD69+ NK cells. C) Bar graphs shows the amount of IFN-γ (pg/ml) secreted by activated NK cells in DC+NK (black bars), NK+IL-12 (white bars) and NK+DC supernatant (gray bars) cultures. D) Bar graphs show IL-12 production in DC-NK co-cultures. B–C–D) Data are expressed as mean ± SEM (n=3-4, n denotes number of individual donors). * p<0.05, relative to Alum. A indicates additive effect.
3.4. Human MoDC-NK mediated T cell polarization in response to selected TLR agonists
After exposure to pathogens, the innate immune cells initiate the differentiation of naïve T cells to distinct effector T cells [28, 29]. Therefore, we next compared the frequency of effector memory CD4 and CD8 T cells generated when naïve T cells co-cultured with the DC and NK cells were exposed to Alum or selected TLR combinations. We observed minimal differences in percentages of central memory CSFE−CCR7+ CD4 and CD8 T cells developed in the primary co-cultures (Figure 6A–B). However, Alum treated co-culture produced the highest percentage (47%) of effector memory CFSE−CCR7− CD4 T cells while TLR4+8 treated co-culture produced the highest percentage (65%) of effector memory CFSE−CCR7− CD8 T cells (Figure 6A–B). In addition, when central and effector memory T cells from the primary co-cultures were restimulated after a period of resting, significantly higher percentages of IFN-γ producing effector CD8 T cells developed from co-cultures that were originally treated with TLR3+8 or TLR4+8 combinations when compared with Alum co-culture (Figure 6C–D). Interestingly, we found no correlation between the frequency of effector Th1 and Tc1 cells and their production of IFN-γ in the co-culture supernatants. Indeed, TLR3+8 or TLR4+8 co-cultures produced significantly higher amount of IFN-γ, 3 and 8 fold respectively when compared with Alum co-culture (Figure 6C–E). Although both TLR3+8 or TLR4+8 co-cultures generated similar frequencies of Th1 and Tc1 effector cells, the effector cells from the latter co-culture produced 2-fold higher IFN-γ in the supernatant (Figure 6C–E). Overall, our data suggest the TLR4 and TLR8 acted in synergy and effectively optimized DC-NK mediated polarization of naïve T cells into IFN-γ producing effector Th1 and Tc1 cells.
Fig 6.
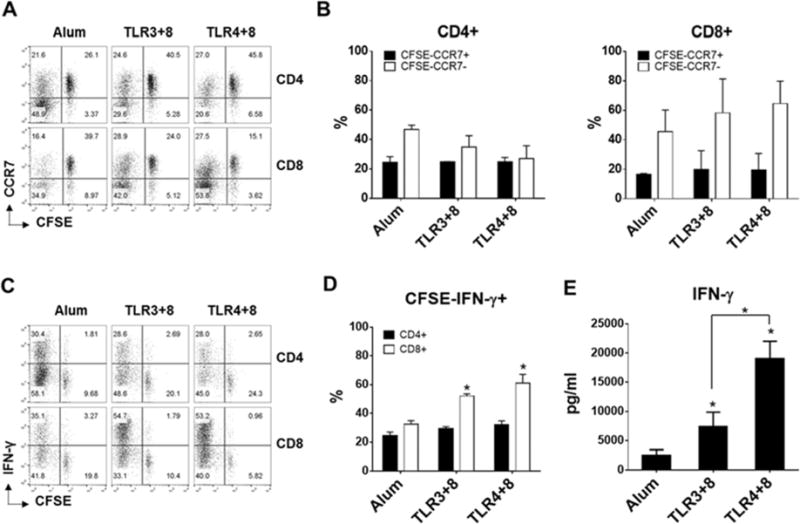
DC, NK, and naïve T cells exposure to aluminum salt or indicated TLR agonists. A–B) Flow plots (A) and bar graphs (B) show the frequency of naïve (CCR7+CFSE+), central memory (CCR7+CFSE−) and effector memory (CCR7−CFSE−) CD4+ and CD8+ T cells generated in the primary co-cultures. C–D) Frequency of IFN-γ producing effector CD4 and CD8 T cells detected within CFSE negative expanded T cells in secondary cultures. E) IFN-γ released in the supernatants of secondary cultures. A–C) One representative flow cytometry data is shown. B–D–E) Data are expressed as mean ± SEM (n=3, n denotes number of individual donors). * p<0.05, relative to Alum. Horizontal bracket denotes the significant difference between indicated samples.
4. Discussion
In the present study, we have evaluated and sequentially selected the TLR agonists that displayed the highest adjuvant effect for the induction of DC maturation, DC-NK crosstalk, and Th1/Tc1 effector cells. Our data shows that TLR3, TLR4, TLR7/8 and TLR8 compared to others were the most effective agonists to induce maturational markers and cytokines production by DCs. In addition, TLR3+8 and TLR4+8 were the combinations that had the highest synergistic effects on IL-12 secretion by DCs and additively promoted IFN-γ production during DC-NK interaction. Finally, we observed that the TLR4+8 combination optimally triggered DC-NK mediated polarization of naïve T cells into IFN-γ producing Tc1 effector cells.
TLRs signal through pathways that involve distinct adaptor molecules, leading to the activation of different transcription factors. These transcription factors (NF-kB, IRF3, IRF7, AP-1) induce the expression of genes coding for antigen presenting molecules, costimulatory molecules, cytokines and chemokines that play a key role in the priming, expansion and polarization of immune cells. The molecular mechanisms by which the single or combination of TLR agonists differentially regulate the corresponding genes in DCs could be dependent on expression of TLRs, adaptor molecules, signaling pathways and duration of signaling [30]. We have shown that TLR5, TLR7, and TLR9 compared to other TLR agonists were less effective in inducing the expression of antigen presenting and costimulatory molecules on DC and their production of cytokines (Figures 1 & 2). One possible explanation is that MoDC express relatively lower levels of TLR5, TLR7, and TLR9 when compared to TLR1-4 [31]. Another possible explanation is that TLR5, TLR7, and TLR9 use the myeloid differentiation primary-response gene 88-dependent (MyD88) to activate NF-kB while TLR2 and TLR4 recruit two adaptor proteins, the Toll/IL-R (TIR)-domain-containing adaptor protein (TIRAP) and MyD88 to activate NF-kB and AP-1 for induction of inflammatory cytokines. Indeed, report indicates that DCs stimulated with ligands for TLR that signal through MyD88/TIRAP produce higher amounts of IL-12 [32]. TLR3 as well as TLR4 uses TRIF to trigger an alternative pathway leading to activation of NF-kB and AP-1 for induction of inflammatory cytokines [30], suggesting that the concomitant activation of NF-kB and AP-1 transcription factors also contribute to differential expression of the costimulatory and cytokines genes in DC.
Our data showed that TLR8 is one of the most effective single agonist to induce DC maturation (Figures 1 & 2). Neither the expression level of TLR8 nor the recruitment of adaptor protein for activation of NF-kB can explain this observation. Indeed, in one study MoDCs expressed much higher level of TLR8 than TLR7 [33], while in another study they expressed lower level of TLR8 than other TLRs except TLR9 [31]. In addition, TLR8 signaling depends on the recruitment of a single adaptor protein MyD88 that culminates in NF-kB activation [30]. Nevertheless, our observation is consistent with previous studies [33, 34] demonstrating that TLR8 agonist expressed higher levels of all maturational markers and IL-12 production than TLR7-activated DCs derived from CD34+ stem cells. The difference in IL-12 production between TLR7 and TLR8 could be explained by the previous observation that IL-12p35 mRNA was up-regulated only by TLR8 but not TLR7 [33]. Since high levels of proinflammatory cytokines require NF-kB activation, it needs to be determined whether TLR8 compared to other TLRs is a more effective inducer of NF-kB in innate immune cells. Using HEK cells transfected with either TLR8 or TLR7, Gordon et al., observed that TLR8 compared to TLR7 agonist is a better activator of NF-kB [34]. Our data is in agreement with previous studies [19, 20] reporting that TLR combinations did not increase the expression of antigen presenting and costimulatory molecules over the expression induced by single TLR agonists (Figures 3 & 4). It has been shown however that certain combinations of TLR ligands act in synergy to enhance the release of cytokines, in particular IL-12 by MoDC and PBMCs [17–20, 35]. We observed that combinations of TLR3, TLR4, TLR7/8, and TLR8 had noticeable synergy for induction of cytokines (Figure 4), probably due to sustained signaling provided by dual TLR engagement [19]. Specifically, MoDC produced the highest level of IL-12 when TLR4+8 were combined followed by when TLR3+8 were combined (Figure 4). This may be because TLR3 activates the Toll/IL-R (TIR)-domain-containing adaptor protein inducing interferon-β (TRIF) pathway and the stimulation of TLR8 activates MyD88 pathway. TLR4 acts through both pathways. The combination of TRIF-associated TLR ligands with MyD88-associated TLR-ligands induces a cooperation between the two signal-transduction pathways downstream of MyD88 and TRIF that may results in synergistic effects [36]. This may be the result of direct influence of TLR3 and to higher extent TLR4 agonist in inducing the higher expression of TLR8 on MoDCs and make them more responsive to TLR8 agonist. For example, it has been shown that TLR7, which is less present on MoDC under normal conditions, is dramatically upregulated after stimulation by TLR4 agonist LPS [18, 37]. Whether TLR3 and TLR4 stimulation of MoDC will also influence their expression of TLR8 needs to be determined. Finally, we found that TLR7/8 and TLR8 combination has less-than-additive effects on proinflammatory cytokines production by MoDCs (Figure 4). The reason for this could be that TLR7/8 competed with, thereby preventing, the binding of TLR8 agonist to its receptor and reduced its effect on cytokines production because TLR8 agonist is more effective than TLR7/8 when it used alone (Figure 2).
DC and NK cells interact with each other through soluble factors and cell-cell contact [38]. Our data shows that TLR4+8 combination not only increased the percentage of activated NK cells during DC-NK crosstalk, but it additively increased the production of IFN-γ by NK cells (Figure 5C). This is consistent with the ability of this TLR agonist combination to stimulate the highest amount of IL-12 secretion by DCs (Figure 5D), which demonstrated to be a central soluble factor for induction of IFN-γ by NK cells [39, 40]. Our data also shows that the adjuvant effects of TLR agonists for promoting DC mediated activation of NK cells was more pronounced during DC-NK crosstalk since addition of exogenous IL-12 or DC-conditioned media to NK cells treated with TLR agonists in the absence of DCs suboptimally activated them (Figure 5). It was shown that PBMC depleted of CD3, CD4, CD14 and DR expressed CD69 following exposure to IL-12 alone, or IL-12 and TLR3 agonist [41]. We also observed that NK cells treated with TLR agonists in the presence of IL-12 or DC-conditioned media upregulate CD69 but only moderately when compared to NK cells co-cultured with DCs (Figure 5A, B). The expression level of CD69 on NK cells observed in these studies could be partially due to differences in experimental settings. Our observation is similar to that of other reports demonstrating that MoDC, matured by microbial stimuli, induced expression of CD69 on NK cells, which was dependent on cell-to-cell contact but independent from endogenous production of IL-12 or IL-2 [42, 43].
Other interactions that promote NK cell production of IFN-γ includes CXC3CL1 expressed on DCs with its receptor on NK cells and triggering of activation receptor NKp46 and NKG2D [43, 44]. Whether the capacity of TLR4+8 to promote the most effective DC-NK interaction was also due to expression of CXC3CL1 and other receptor-ligands and/or other soluble factors merit further investigation.
Increased protection from reinfection requires the selection of adjuvants that increase the number of memory T cells. Our data demonstrate that Alum was an effective adjuvant to increase the frequency of effector memory CD4 T cells whereas TLR3+8 and TLR4+8 combinations were more effective to promote effector memory CD8 T cells (Figure 6). Overall, however, TLR4+8 combination showed superior adjuvant activity to polarize the DC-NK mediated differentiation of naïve T cells into both effector memory CD4 and in particular CD8 cells producing IFN-γ (Figure 6). Our findings are consistent with previous study, which demonstrated that the combination of TLR4 and TLR8 improves the expansion of peptide-specific human CD8 T cells in vitro (Bohnenkamp HR et al, 2007). Interestingly, while TLR3+8 and TLR4+8 combinations developed almost the same frequency of total effector memory cells, the effector cells produced in response to TLR4+8 secreted quantitatively much more IFN-γ in the supernatant (Figure 6E). This adjuvant effect of TLR4+8 combination on effector CD4 and CD8 polarization may be directly correlated to high levels of IL-12, IL1β, and TNF-α produced by DCs during co-cultures. Indeed, there is considerable evidence indicating that IL-1β and IL-12 provide a third signal to support clonal expansion and development of CD4 and CD8 T effector memory function in response to antigen, respectively [45].
We also observed that DC alone stimulated with TLR3+8 or TLR4+8 promoted less production of effector memory CD4 and CD8 T cells (data not shown) suggesting the IFN-γ produced by NK cells during DC-NK co-cultures contributed further to DC maturation and consequently more production of effector memory CD4 and particularly CD8 T cells. This finding is consistent with other studies suggesting that early production of IFN-γ by NK cells is important for development CD8 effector T cells (Whitmire JK et al, 2005; Meijuan Zheng at al, 2016).
5. Conclusions
In summary, our results reveal the differences in TLR agonists-induced engagement of innate and adaptive immune components for effective polarization of Th1 and Tc1 effector cells. The results also provide a useful reference when the selection of adjuvants and their combinations is based upon criteria such as DC and NK cell crosstalk and magnitude of desired cellular responses.
Highlights.
TLR3, TLR4, TLR7/8 and TLR8 were the most effective adjuvants to mature DCs.
TLR3+8 and TLR4+8 combinations had the most synergistic and additive effects on IL-12 secretion by DCs and IFN-γ secretion by NK cells, respectively, during DC-NK interaction.
DC-NK-T treated with aluminum salt produced the highest percentage of effector memory Th1 cells
DC-NK-T treated with TLR3+8 and TLR4+8 produced the highest percentage of effector memory Tc1 cells.
TLR4+8 combination was the most effective adjuvants to trigger DC-NK mediated polarization of Tc1 effector memory cells.
Acknowledgments
We thank Dr. Jon R. Inglefield for critical reading of the manuscript.
Funding
This work was supported by National Institute of Allery and Infectious Diseases, National Institute of Health Grant 1R03AI103750-01A1 (to M.N.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no financial conflicts of interest.
References
- 1.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duthie MS, Windish HP, Fox CB, R SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239(1):178–96. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 4.Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 29(17):3341–55. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 6.Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135(3):626–35. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25(1):47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins RJ, Kalsi G, Montalvo-Lugo VM, Sharma M, Wu Y, Muse DD, Sheldon EA, Hampel FC, Lemiale L. Randomized, double-blind, active-controlled study evaluating the safety and immunogenicity of three vaccination schedules and two dose levels of AV7909 vaccine for anthrax post-exposure prophylaxis in healthy adults. Vaccine. 34(18):2096–105. doi: 10.1016/j.vaccine.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc Natl Acad Sci U S A. 111(34):12294–9. doi: 10.1073/pnas.1400478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toussi DN, Massari P. Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands. Vaccines (Basel) 2(2):323–53. doi: 10.3390/vaccines2020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ, de Vries IJ. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother. 59(10):1573–82. doi: 10.1007/s00262-010-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahonen CL, Gibson SJ, Smith RM, Pederson LK, Lindh JM, Tomai MA, Vasilakos JP. Dendritic cell maturation and subsequent enhanced T-cell stimulation induced with the novel synthetic immune response modifier R-848. Cell Immunol. 1999;197(1):62–72. doi: 10.1006/cimm.1999.1555. [DOI] [PubMed] [Google Scholar]
- 14.Bohnenkamp HR, Papazisis KT, Burchell JM, Taylor-Papadimitriou J. Synergism of Toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type 1 responses. Cell Immunol. 2007;247(2):72–84. doi: 10.1016/j.cellimm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Hackstein H, Knoche A, Nockher A, Poeling J, Kubin T, Jurk M, Vollmer J, Bein G. The TLR7/8 ligand resiquimod targets monocyte-derived dendritic cell differentiation via TLR8 and augments functional dendritic cell generation. Cell Immunol. 271(2):401–12. doi: 10.1016/j.cellimm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Jensen SS, Gad M. Differential induction of inflammatory cytokines by dendritic cells treated with novel TLR-agonist and cytokine based cocktails: targeting dendritic cells in autoimmunity. J Inflamm (Lond) 7:37. doi: 10.1186/1476-9255-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007;68(10):813–22. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Lombardi V, Van Overtvelt L, Horiot S, M P. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-gamma, and IL-17A by naive CD4+ T cells. J Immunol. 2009;182(6):3372–9. doi: 10.4049/jimmunol.0801969. [DOI] [PubMed] [Google Scholar]
- 19.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paustian C, Caspell R, Johnson T, Cohen PA, Shu S, Xu S, Czerniecki BJ, Koski GK. Effect of multiple activation stimuli on the generation of Th1-polarizing dendritic cells. Hum Immunol. 72(1):24–31. doi: 10.1016/j.humimm.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Spranger S, Javorovic M, Bürdek M, Wilde S, Mosetter B, Tippmer S, Bigalke I, Geiger C, Schendel DJ, F B. Generation of Th1-polarizing dendritic cells using the TLR7/8 agonist CL075. J Immunol. 2010;185(1):738–47. doi: 10.4049/jimmunol.1000060. [DOI] [PubMed] [Google Scholar]
- 22.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 23.Mazzoni A, Segal DM. Controlling the Toll road to dendritic cell polarization. J Leukoc Biol. 2004;75(5):721–30. doi: 10.1189/jlb.1003482. [DOI] [PubMed] [Google Scholar]
- 24.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1(4):311–6. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 25.Sandor F, Buc M. Toll-like receptors. II. Distribution and pathways involved in TLR signalling. Folia Biol (Praha) 2005;51(6):188–97. [PubMed] [Google Scholar]
- 26.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27(1):49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Morandi B, Bougras G, Muller WA, Ferlazzo G, Munz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol. 2006;36(9):2394–400. doi: 10.1002/eji.200636290. [DOI] [PubMed] [Google Scholar]
- 28.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3(12):984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 29.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106(3):263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 30.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 34(5):637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170(10):5165–75. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 32.Krummen M, Balkow S, Shen L, Heinz S, Loquai C, Probst HC, Grabbe S. Release of IL-12 by dendritic cells activated by TLR ligation is dependent on MyD88 signaling, whereas TRIF signaling is indispensable for TLR synergy. J Leukoc Biol. 88(1):189–99. doi: 10.1189/jlb.0408228. [DOI] [PubMed] [Google Scholar]
- 33.Larange A, Antonios D, Pallardy M, Kerdine-Romer S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. J Leukoc Biol. 2009;85(4):673–83. doi: 10.1189/jlb.0808504. [DOI] [PubMed] [Google Scholar]
- 34.Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174(3):1259–68. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh TK, Mickelson DJ, Solberg JC, Lipson KE, Inglefield JR, Alkan SS. TLR-TLR cross talk in human PBMC resulting in synergistic and antagonistic regulation of type-1 and 2 interferons, IL-12 and TNF-alpha. Int Immunopharmacol. 2007;7(8):1111–21. doi: 10.1016/j.intimp.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7(3):179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 37.Severa M, Remoli ME, Giacomini E, Annibali V, Gafa V, Lande R, Tomai M, Salvetti M, Coccia EM. Sensitization to TLR7 agonist in IFN-beta-preactivated dendritic cells. J Immunol. 2007;178(10):6208–16. doi: 10.4049/jimmunol.178.10.6208. [DOI] [PubMed] [Google Scholar]
- 38.Thomas R, Yang X. NK-DC Crosstalk in Immunity to Microbial Infection. J Immunol Res. 2016:6374379. doi: 10.1155/2016/6374379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Girart MV, Fuertes MB, Domaica CI, Rossi LE, Zwirner NW. Engagement of TLR3, TLR7, and NKG2D regulate IFN-gamma secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J Immunol. 2007;179(6):3472–9. doi: 10.4049/jimmunol.179.6.3472. [DOI] [PubMed] [Google Scholar]
- 40.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175(3):1636–42. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 41.Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci U S A. 2004;101(27):10116–21. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195(3):327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pallandre JR, Krzewski K, Bedel R, Ryffel B, Caignard A, Rohrlich PS, Pivot X, Tiberghien P, Zitvogel L, Strominger JL, Borg C. Dendritic cell and natural killer cell cross-talk: a pivotal role of CX3CL1 in NK cytoskeleton organization and activation. Blood. 2008;112(12):4420–4. doi: 10.1182/blood-2007-12-126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Draghi M, Pashine A, Sanjanwala B, Gendzekhadze K, Cantoni C, Cosman D, Moretta A, Valiante NM, Parham P. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178(5):2688–98. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 45.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 22(3):333–40. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


