Figure 1. The multi-functional role of CNS pericytes at the neurovascular unit (NVU).
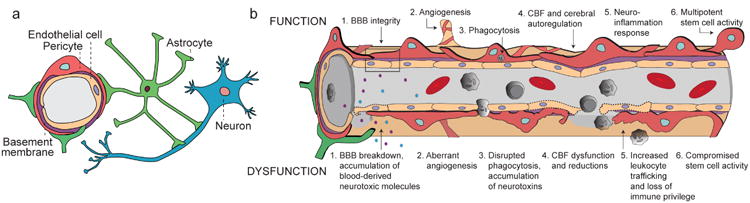
(a) A simplified NVU diagram showing the interactive cellular network at the level of brain capillaries that comprises vascular cells (e.g., pericytes and endothelial cells), glial cells (e.g., astrocytes), and neurons. Intricate cell-cell communication and signal transduction mechanisms of NVU cell types are highly controlled to regulate numerous functions in the CNS. (b) Under physiological conditions (top row), pericytes regulate 1) blood-brain barrier (BBB) integrity, i.e., tight/adherens junctions and transcytosis across the BBB; 2) angiogenesis, i.e. microvascular remodeling, stability, and architecture; 3) phagocytosis, i.e., clearance of toxic metabolites from the CNS; 4) cerebral blood flow (CBF) and capillary diameter; 5) neuroinflammation, i.e., leukocyte trafficking into the brain; and 6) multipotent stem cell activity. Pericyte dysfunction (bottom row) is characterized by 1) BBB breakdown causing leakage of neurotoxic blood-derived molecules into the brain (e.g., fibrinogen, thrombin, plasminogen, erythrocyte-derived free iron, and anti-brain antibodies); 2) aberrant angiogenesis; 3) impaired phagocytosis causing CNS accumulation of neurotoxins; 4) CBF dysfunction and ischemic capillary obstruction; 5) increased leukocyte trafficking promoting neuroinflammation; and 6) impaired stem cell-like ability to differentiate into neuronal and hematopoietic cells. Pericyte dysfunction is present in numerous neurological conditions and can contribute to the disease pathogenesis.
