Figure 2. PDGF-BB/PDGFRp signaling in pericytes.
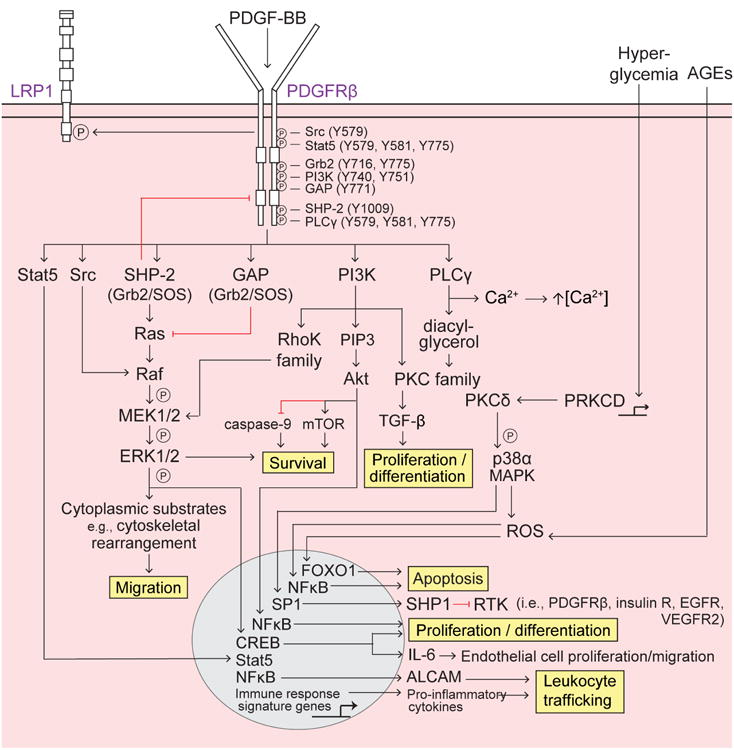
Platelet-derived growth factor-BB (PDGF-BB) secreted by endothelial cells binds to the PDGF receptor-β (PDGFRβ) on pericytes, causing receptor dimerization, autophosphorylation, and activation. Several Src homology 2 (SH2) domain-containing proteins (Src, Stat5, Grb2, phosphatidyl inositol 3-phosphate (PI3K), GTPase activating protein (GAP), SH2 tyrosine phosphatase (SHP-2), and phospholipase Cγ (PLCγ) bind to distinct phosphorylated (P) tyrosine (Y) residues SH2 domain-containing proteins bound to PDGFRβ differentially activate downstream signaling pathways to regulate pericyte survival, migration, apoptosis, proliferation and differentiation, as well as leukocyte trafficking, described as follows: Survival – promoted via PI3K-Akt activation of mammalian target of rapamycin (mTOR) and inhibition of caspase-9 and the SHP-2-mediated MAPK pathway; Migration – SHP-2-mediated MAPK pathway promotes cytoskeletal rearrangement and cell migration. Src activated Raf synergistically activates the MAPK pathway whereas GAP inhibition of Ras decreases MAPK signaling; Apoptosis – Extracellular advanced glycation endproducts (AGEs) induce intracellular reactive oxygen species (ROS) and FOXO1-mediated apoptosis, and PRKCD transcriptional expression of protein kinase C-δ (PKCδ) activates p38α MAPK to induce downstream production of ROS and mitochondrial cytochrome c release, resulting in apoptosis; Proliferation and differentiation – promoted by the PI3K pathway, specifically via PKC-TGF-β and PIP3-Akt transcriptional activation of NFκB; Leukocyte trafficking – PDGFRβ regulates pro-inflammatory responses, e.g., peripheral leukocyte trafficking into the CNS, via transcriptional expression of immune response signaling genes (e.g., cytokines and chemokines) and also via Akt-induced activation of NFκB and transcriptional expression of the novel activated leukocyte cell adhesion molecule (ALCAM).
