Abstract
Pregnancy predisposes women to disproportionate morbidity and mortality from influenza infections. This is true for both seasonal epidemics as well as occasional pandemics. Inactivated yearly influenza vaccines are the best available method of disease prevention and are recommended for all pregnant women in any trimester of pregnancy and postpartum. Oseltamivir (Tamiflu®) is currently the first-line recommended and most commonly used pharmaceutical agent for influenza prophylaxis and treatment. Oseltamivir has been demonstrated to prevent disease among exposed individuals, as well as to shorten the duration of illness and lessen the likelihood of complications among those infected. The physiologic adaptations of pregnancy may alter the pharmacokinetics and pharmacodynamics of this important drug. Updated evidence regarding these potential alterations, current knowledge gaps, and future investigative directions is discussed.
Keywords: Influenza, Pregnancy, Oseltamivir, Pharmacology
Background
It is well documented and accepted that pregnant women have an increased risk of untoward outcomes (higher morbidity, hospitalization, and mortality rates) from influenza infection when compared to the general adult population. This increased risk includes infection due to both seasonal outbreaks as well as the occasional pandemic.1–4 The concern that pregnant women would have worse clinical outcomes than non-pregnant women or men was clearly validated during the recent 2009 H1N1 pandemic and unfortunately continues to be noted since 2009.5–7 The literature also suggests that the heightened risks from influenza in pregnancy increase as pregnancy progresses, peaking in the late 2nd trimester and 3rd trimester and continuing into the early postpartum period.1–7 It is believed that a combination of relative immune compromise and anatomic (i.e., size of abdomen relative to chest cavity and its effect on respiratory dynamics) and physiologic (i.e., respiratory and cardiovascular) alterations associated with pregnancy predispose to these elevated risks.
Mounting evidence also suggests that influenza infection in pregnancy (especially influenza pneumonia) likewise worsens pregnancy outcomes. Multiple investigations have demonstrated higher rates of pre-term birth and compromised fetal growth among women suffering from influenza in pregnancy.8–11 Given these noted complications from influenza in pregnancy, prevention of infection is of paramount importance.
The primary strategy for influenza infection prevention in pregnancy is receipt of the seasonal inactivated vaccine. This intervention is recommended for all pregnant women (lacking contraindication) and is safe to give at any point during the pregnancy.1,12 Recent estimates suggest that despite the clear and well-publicized recommendation for influenza vaccine to all pregnant women, only 45–50% receive the vaccine.13 This is a major improvement from pre-pandemic levels of vaccination, but still falls well below the Healthy People 2020 goal of 80%.14 Concerted efforts to extensively improve these vaccination rates are underway.
Given these heightened risks from influenza in pregnancy and the persistent suboptimal vaccine uptake, as well as persistent influenza vaccine efficacy rates of around 60–70%,1 antiviral medications for both prevention and treatment remain important considerations for obstetric providers. There are 2 distinct classes of anti-influenza antiviral medications available for use, the older adamantane drugs (amantadine and rimantadine) and the newer neuraminidase inhibitor drugs (oseltamivir, zanamivir, and peramivir). Since 2009, the adamantanes are no longer recommended for use due to concerns about antiviral resistance among circulating influenza viruses.15 Currently, the most commonly used antiviral (and primarily recommended) for influenza treatment and prevention is oseltamivir (Tamiflu®). All neuraminidase inhibitor drugs exert their clinical effects by acting as a competitive inhibitor of the influenza viral neuraminidase (NA) enzyme, which acts on sialic acid residues. Sialic acid is present on the surface receptors of host cells, and blocking this enzyme prevents new infectious virus from being released from infected host cells, thus limiting and/or halting viral spread. Oseltamivir is currently recommended for the prevention of influenza among exposed individuals given its apparent ability to reduce rates of infection by 70–90% when used once daily as directed.15,16 When given for influenza prevention, the dose recommendation is one 75-mg tablet taken daily for 7 days after exposure.15
Once women develop symptoms consistent with influenza (fever, sore throat, cough, malaise, fatigue, prostration, shortness of breath, etc.), antiviral treatment becomes an important consideration. The recommendation is to begin therapy as soon as possible after symptom onset, ideally within 48 h.15 The majority of data for use of these antivirals in pregnancy is with oseltamivir, although the overall data quantity is relatively limited. The safety data that are available, in addition to growing clinical experience, provide evidence for use in pregnancy.17–22 Importantly, oseltamivir has been demonstrated in large systematic reviews of the general population to lessen the duration of infection and hasten recovery among adults and children with influenza.16,23 Additional systematic review data among the general population also suggest that it may also lessen the chances of severe illness and hospitalization among infected individuals.24 In addition to data from systematic review in the general population, recent observational data of clinical use of oseltamivir in pregnancy during the 2009 H1N1 influenza pandemic also support these findings in terms of effectiveness and ability to minimize severe illness with attention to earlier initiation of therapy.6,25 The probability of severe disease appears to increase as the interval from symptom onset to treatment increases. Creanga et al.25 demonstrated that the percentages of pregnant patients in New York City with influenza having severe illness increased from 3% if treatment was started <48 h after symptom onset to 44% if treatment was started more than 5 days after symptom onset (p = 0.002). Likewise, a separate U.S. investigation of 788 pregnant women with 2009 H1N1 demonstrated a significantly elevated relative risk of 6.0 (95% C.I.: 3.5–10.6) for admission to the intensive care unit (ICU) for women treated more than 4 days after symptom onset (compared to less than 48 h).6 Clinical experience also confirms these findings from observational studies.
Key to optimal use of oseltamivir (as well as any therapeutic agent) in pregnancy is the complete understanding of the pharmacokinetics (PK) and pharmacodynamics (PD) of the medication as well as the impact of physiologic alterations in pregnancy which invariably modulate drug exposure.26 Important pharmacologic alterations in pregnancy among numerous therapeutic agents have been noted for other conditions.27–30 In addition to the potential for alteration in therapeutic efficacy with pharmacokinetic changes, use of antimicrobial agents carries the additional public health consideration of potential for drug resistance from subtherapeutic levels of drug. Evolving data, including previously published as well as ongoing efforts within the Obstetric-Fetal Pharmacologic Research Unit Network (OPRU), is beginning to inform the impact that pregnancy-induced physiologic alterations have on this very important antiviral drug.
The basic pharmacology of oseltamivir
Oseltamivir phosphate (OP) is an orally administered prodrug that is well absorbed. It then undergoes significant conversion by hepatic carboxylesterases (carboxylesterase-1) to the single active metabolite oseltamivir carboxylate (OC) (Fig.).31 Less than 5% of the ingested pro-drug is excreted unchanged in the urine. The absolute bioavailability of the active metabolite after oral administration is approximately 80%. The pharmacokinetics (PK) of OC appears to be linear across many dose ranges. Peak plasma concentrations are achieved after approximately 2–4 h. After systemic distribution, oseltamivir carboxylate is exclusively eliminated by renal excretion, with an elimination half-life (t1/2) of 6–10 h. There is limited potential for drug-drug interactions with OC (Table 1).
Fig.
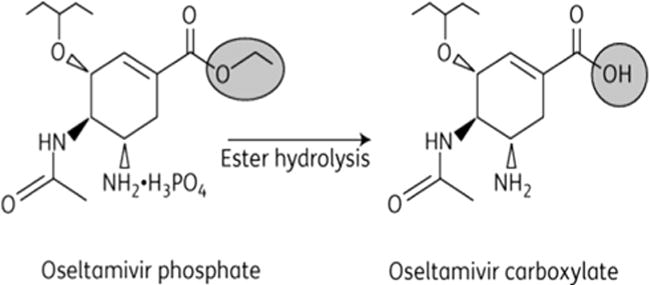
Structure of Oseltamivir parent drug (OP) and active metabolite (OC).
Table 1.
Pharmacokinetics properties of oseltamivir phosphate (OP, parent drug) and oseltamivir carboxylate (OC, active metabolite) among pregnant and non-pregnant women.19,31
| Drug and parameter | Non-pregnant | Pregnant |
|---|---|---|
| OP (parent drug) | ||
| Bioavailability | 80% | |
| AUCa (ng/mL/h) | 157 (±54) | 164 (±51) |
| CL/Fb (L/h) | 543 (±225) | 501 (± 160) |
| Vz/Fc (L) | 1768 (±1445) | 2108 (± 1366) |
| T1/2d(h) | 3 (±4.1) | 3.1 (± 2.1) |
| OC (active metabolite) | ||
| AUC (ng/mL/h) | 4719 (± 1263) | 3460 (± 1350) |
| CL/F (L/h) | 17 (± 4.7) | 24.5 (± 8.4) |
| Vz/F (L) | ||
| T1/2 (h) | 7.1 (± 1.9) | 6.2 (± 1.6) |
Area under the plasma concentration curve.
Apparent oral clearance.
Apparent volume of distribution.
Apparent half-life.
Given the known effects of pregnancy on the gastrointestinal, hepatic, and renal systems, biologic plausibility exists for altered PK of oseltamivir with subsequent implications for dosing recommendations. Moreover, insights into whether (and the extent to which) oseltamivir crosses the placenta could also have relevant implications in terms of pharmacokinetic profile, maternal, fetal, and neonatal effects. It is important to note that although the increased risks of influenza in pregnancy have been known for more than a century, none of the greater than 15 published studies (primarily in healthy Caucasian volunteers) on the PK of oseltamivir included pregnant women.
The relationship between PK of the drug and the clinical effects noted in patients suffering from influenza is not well delineated. Limited investigations in the general adult population have attempted to correlate the PK of oseltamivir carboxylate with important pharmacodynamic (PD) endpoints including efficacy. Given the severity of influenza in certain populations (including pregnant women), the everpresent and important potential for antiviral resistance, and the desire to target appropriate therapy to the specific patient, this relationship becomes important. This is especially true among infected patients, who could exhibit changes in the PK of the drug (and therefore clinical effects). McSharry et al. have suggested approximating such a relationship using an in-vitro model system. They proposed that the variable AUC24:IC50 might provide a useful efficacy end point to allow for better PD approximations and correlations with PK.32 The robustness of this proposed variable in terms of population clinical effects remains to be elucidated.
Oseltamivir pharmacology in pregnancy
As mentioned, limited data exist evaluating the effects of pregnancy on the PK of oseltamivir. Both of the available studies used an “opportunistic” study design by enrolling pregnant women already on treatment courses of oseltamivir for confirmed and/or suspected influenza infection. This study design can be a very effective way of conducting investigations in pregnancy by taking advantage of the fact that women are already receiving drug, thus minimizing some potential logistical barriers to conducting research on therapeutics in pregnancy. However, this design does pose some challenges due to the already present dosing schedule with limited ability to control for differences in dosing schedules.33 Nevertheless, insights into potential effects have been and can continue to be gained from this approach.
Greer et al.34 studied the PK of oseltamivir among pregnant women with the primary goal of comparing the potential for trimester-specific differences in drug metabolism. Overall, 30 women (10 per trimester) were enrolled into this opportunistic study, and all were taking either the usual adult recommended treatment dose of 75 mg twice daily (b.i. d.) or the prophylactic dose of 75 mg once daily. All blood sampling occurred before and after the 1st dose of drug only. The investigators found no significant differences in the common PK parameters (Cmax, Tmax, and AUC0-12) for the parent pro-drug between trimesters. They did detect a significant lengthening of half-life (T1/2) for women in the 1st trimester compared with later in pregnancy (4 h vs. approximately 2 h).34 Similarly, when they looked at the active metabolite OC, they noted similar PK by trimester with the only significant difference detected in Cmax (higher in 3rd trimester). The authors noted that despite these small fluctuations all values were within the pharmacologic range that was predicted to be clinically effective against circulating strains of influenza. They thus concluded that the PK of oseltamivir (and its active metabolite) was not significantly different by trimester.34
In contrast, Beigi et al.19 (as part of the Obstetric-Fetal Pharmacologic Research Unit-OPRU Network) studied both pregnant and non-pregnant women within the same study. A total of 16 pregnant women already on therapy were enrolled along with 23 non-pregnant controls (not already on therapy; took oseltamivir for study purposes) in an attempt to offer comparative data within the same trial. The pregnant cohort was made up of 3 women in the 1st trimester, 9 in the 2nd, and 4 in the 3rd. Of the 16 pregnant women, 14 (88%) were on oseltamivir for treatment (12/14 on b.i.d. dosing and 2 on t.i.d. dosing, per physician discretion) and 2 were receiving once daily 75-mg prophylaxis doses. Despite these differences in dosing within the pregnant cohort, all women in both cohorts underwent intensive PK sampling after being on oseltamivir therapy for at least 48 h. Dose-adjusted pharmacokinetic analysis was used in this investigation to control for these noted differences in dosing. The physiologic changes of pregnancy resulted in an approximate 30% lower exposure (p = 0.007) to the active metabolite OC when compared to the non-pregnant women. This was noted to likely coincide with the increased apparent oral drug clearance from the enhanced renal elimination among the pregnant subjects.19 The authors also note in this study that the renal excretion of oseltamivir exceeds the glomerular filtration rate. This suggests that renal tubular secretion via an organic anion transporter (OAT-1 specifically) contributes, as has been seen in other investigations.29 It is possible that pregnancy produces an up-regulation of this transporter and may be responsible for this phenomenon. While the precise pharmacodynamic impact (and direct clinical implications) of the findings from this investigation is unclear, these data suggest that altered dosing schedules among pregnant women may be necessary to better approximate the drug exposure among non-pregnant subjects. While compelling, definitive recommendations regarding pregnancy-specific dosing guidance cannot be made based on these data given the small sample size.19,33 Ongoing and future investigations will strive to provide data that can permit more accurate assessments enabling more definitive recommendations on dosing schedules in pregnancy.
Attention has also been focused on the potential for oseltamivir and its active metabolite oseltamivir carboxylate (OC) to cross the placenta and be detected in the fetus. Ex-vivo placental transfer models have primarily been used by different investigators to begin to address this important question.35–37 Additionally, a single recent clinical case report has also addressed this issue.38 Despite use of differing methodologies, all ex-vivo placental transfer model investigations have suggested that OC can and will traverse the placenta and will likely be detected in the fetal compartment, however, at predicted significantly lower concentrations than that seen in the maternal circulation.35–37 The precise impact of the findings from these models remains to be clinically validated. Meijer et al.38 have reported a single case of a 2009 H1N1-infected mother that underwent cesarean delivery due to illness and had umbilical cord blood obtained at the time of delivery. This single investigation detected higher levels of both parent and active drug in the fetal cord blood than might be predicted from the ex-vivo models. The absolute values however were 4-fold lower than the maternal levels and are of questionable clinical significance. Based on this one finding, the authors suggest that oseltamivir only be used to treat critically ill pregnant women given the unknown impact of such drug exposure. We do not agree with this recommendation and find it potentially misleading to clinicians, given clear benefit from treatment of pregnant women as well as a lack of any data to suggest fetal/neonatal harm from indicated and necessary maternal therapy. While these data on placental transfer taken together are interesting, there are many unknown variables with this clinical calculation, and it is recommended that the current clinical guidance be followed that all pregnant women exposed to or with early influenza symptomatology be treated with oseltamivir.1,15 Clearly, additional investigations are needed to delineate a definitive understanding of the PK and PD of oseltamivir in pregnant women with influenza. Suggestions for future investigation to improve the understanding of the PK and PD of oseltamivir in pregnancy are listed in Table 2.
Table 2.
Suggestions for future investigations of oseltamivir in pregnancy.
| 1. | Additional PK analysis including well-matched non-pregnant controls |
| 2. | Delineation of PD end points and the impact of pregnancy on these end points |
| 3. | Exposure and outcome analyses |
| 4. | Impact of PK and PD alterations in pregnancy on viral resistance |
| 5. | Attention to the impact of maternal PK and PD alterations on neonatal outcomes |
Conclusions
Oseltamivir is an important drug for use in pregnancy given the increased risks of influenza in pregnancy and the potential for oseltamivir to substantially mitigate these heightened risks. Early pharmacologic investigations begin to delineate the PK of this drug in pregnancy, and these insights are being used to hypothesize on the precise clinical impact of these findings. While these findings are intriguing, ongoing and future investigation must strive to improve our knowledge of the PK and PD to enable more informed clinical use of this important antiviral therapy.
Acknowledgments
This work was supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD047905, HD047891, and HD047892), and by the Office of Research on Women’s Health, NIH, USA.
References
- 1.CDC. Prevention and control of influenza with vaccines. Recommendations of the advisory committee on immunization practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 2.Irving WL, James DK, Stephenson T, et al. Influenza virus infection in the second and third trimesters of pregnancy: a clinical and seroepidemiological study. BJOG. 2000;107:1282–1289. doi: 10.1111/j.1471-0528.2000.tb11621.x. [DOI] [PubMed] [Google Scholar]
- 3.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094–1102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 4.Beigi RH. Clin Obstet Gynecol. 2012;55(4):914–926. doi: 10.1097/GRF.0b013e31827146bd. [DOI] [PubMed] [Google Scholar]
- 5.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 6.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. J Am Med Assoc. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louie JK, Acosta M, Jamieson DJ, Honein MA California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 8.Sheffield JS, Cunningham FG. Community-acquired pneumonia in pregnancy. Obstet Gynecol. 2009;114(4):915–922. doi: 10.1097/AOG.0b013e3181b8e76d. [DOI] [PubMed] [Google Scholar]
- 9.Naresh A, Fisher BM, Hoppe KK, et al. A multicenter cohort study of pregnancy outcomes among women with laboratory-confirmed H1N1 influenza. J Perinatol. 2013;33(12):939–943. doi: 10.1038/jp.2013.110. [DOI] [PubMed] [Google Scholar]
- 10.Hewagama S, Walker SP, Stuart RL, et al. 2009 H1N1 influenza A and pregnancy outcomes in Victoria, Australia. Clin Infect Dis. 2010;50(5):686–690. doi: 10.1086/650460. [DOI] [PubMed] [Google Scholar]
- 11.Mendez-Figueroa H, Raker C, Anderson BL. Neonatal characteristics and outcomes of pregnancies complicated by influenza infection during the 2009 pandemic. Am J Obstet Gynecol. 2011;204(6 suppl 1):S58–S63. doi: 10.1016/j.ajog.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ACOG Committee on Obstetric Practice. ACOG Committee Opinion No 468: influenza vaccination during pregnancy. Obstet Gynecol. 2010;116:1006–1007. doi: 10.1097/AOG.0b013e3181fae845. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) Pregnant women and flu vaccination, Internet Panel Survey, United States, November 2013. 〈 http://www.cdc.gov/flu/fluvaxview/pregnant-women-nov2013.htm〉; Accessed 20.01.14.
- 14.Healthy People 2020. Topics and objectives: immunizations and infectious diseases, IID-12.10. 〈 http://www.healthypeople.gov/2020/topics-objectives2020/objectiveslist.aspx?topicId=23〉; Accessed 20.01.14.
- 15.CDC. 2011–2012 influenza antiviral medications: summary for clinicians. 〈 http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm〉; Accessed 20.01.14. [PubMed]
- 16.Jefferson T, Demicheli V, Deeks J, Rivetti D. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev. 2000;(2):CD001265. doi: 10.1002/14651858.CD001265. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Nakajima K, Murashima A, Garcia-Bournissen F, Koren G. Safety of neuraminidase inhibitors against novel influenza A (H1N1) in pregnant and breasfeeding women. CMAJ. 2009;181(1–2):55–58. doi: 10.1503/cmaj.090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer LG, Sheffield JS, Rogers VL, Roberts SW, Mcintire DD, Wendell GD. Maternal and neonatal outcomes after antepartum treatment of influenza with antiviral medications. Obstet Gynecol. 2010;115:711–716. doi: 10.1097/AOG.0b013e3181d44752. [DOI] [PubMed] [Google Scholar]
- 19.Beigi RH, Han K, Venkataramanan R, et al. Pharmacokinetics of oseltamivir among pregnant and nonpregnant women. Am J Obstet Gynecol. 2011;204(6 suppl 1):S84–S88. doi: 10.1016/j.ajog.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donner B, Viswanathan N, Hoffman G. Safety of oseltamivir in pregnancy: a review of preclinical and clinical data. Drug Saf. 2010;33(8):631–642. doi: 10.2165/11536370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Xie HY, Yasseen AS, Xie RH, et al. Infant outcomes among pregnant women who used oseltamivir for treatment of influenza during the H1N1 epidemic. Am J Obstet Gynecol. 2013;208(293):e1–e7. doi: 10.1016/j.ajog.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Saito S, Minakami H, Nakai A, Unno N, Kubo T, Yoshimura Y. Outcomes of infants exposed to oseltamivir or zanamivir in utero during pandemic (H1N1) 2009. Am J Obstet Gynecol. 2013;209(130):e1–e9. doi: 10.1016/j.ajog.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Matheson NJ, Symmonds-Abrahams M, Sheikh A, Shepperd S, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database Syst Rev. 2003;3:CD002744. doi: 10.1002/14651858.CD002744. [DOI] [PubMed] [Google Scholar]
- 24.Hsu J, Santesso N, Mustafa R, et al. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2012;156(7):512–524. doi: 10.7326/0003-4819-156-7-201204030-00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creanga AA, Johnson TF, Graitcer SB, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115(4):717–726. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 26.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44(10):989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 27.Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85(6):607–614. doi: 10.1038/clpt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchanan ML, Easterling TR, Carr DB, et al. Clonidine pharmacokinetics in pregnancy. Drug Metab Dispos. 2009;37(4):702–705. doi: 10.1124/dmd.108.024984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrew MA, Easterling TR, Carr DB, et al. Amoxicillin pharmacokinetics in pregnant women: modeling and simulations of dosage strategies. Clin Pharmacol Ther. 2007;81(4):547–556. doi: 10.1038/sj.clpt.6100126. [DOI] [PubMed] [Google Scholar]
- 30.Eval S, Easterling TR, Carr DB, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38:833–840. doi: 10.1124/dmd.109.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutkowski R, Thakrar B, Froehlich E, Suter P, Oo C, Ward P. Safety and pharmacology of oseltamivir in clinical use. Drug Safety. 2003;26(11):787–801. doi: 10.2165/00002018-200326110-00004. [DOI] [PubMed] [Google Scholar]
- 32.McSharry JJ, Weng Q, Brown A, Kulaway R, Drusano GL. Prediction of the pharmacodynamically linked variable of oseltamivir carboxylate for influenza A virus using an in vitro hollow-fiber infection model system. Antimicrob Agents Chemother. 2009;53:2375–2381. doi: 10.1128/AAC.00167-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirochnick M, Clarke D. Oseltamivir pharmacokinetics in pregnancy: a commentary. Am J Obstet Gynecol. 2011;204:S94–S95. doi: 10.1016/j.ajog.2011.02.043. [DOI] [PubMed] [Google Scholar]
- 34.Greer LG, Leff RD, Laibl Rogers V, et al. Pharmacokinetics of oseltamivir according to trimester of pregnancy. Am J Obstet Gynecol. 2011;204:S89–S93. doi: 10.1016/j.ajog.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worley KC, Roberts SW, Bawdon RE. The metabolism and transplacental transfer of oseltamivir in the ex vivo human model. Infect Dis Obstet Gynecol. 2008;2008:927574. doi: 10.1155/2008/927574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berveiller P, Mir O, Vinot C, et al. Transplacental transfer of oseltamivir and its metabolite using the human perfused placental cotyledon model. Am J Obstet Gynecol. 2012;206(92):e1–e6. doi: 10.1016/j.ajog.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Nanovskaya TN, Patrikeeva S, Zhan Y, Hankins GDV, Ahmed MS. Transplacental transfer of oseltamivir carboxylate. J Matern Fetal Neonatal Med. 2012;25(11):2312–2315. doi: 10.3109/14767058.2012.693993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meijer WJ, Bruinse HW, van den Broek MP, Kromdijk W, Wensing AM. Oseltamivir and its active metabolite cross the placenta at significant levels. Clin Infect Dis. 2012;54:1676–1677. doi: 10.1093/cid/cis265. [DOI] [PubMed] [Google Scholar]


