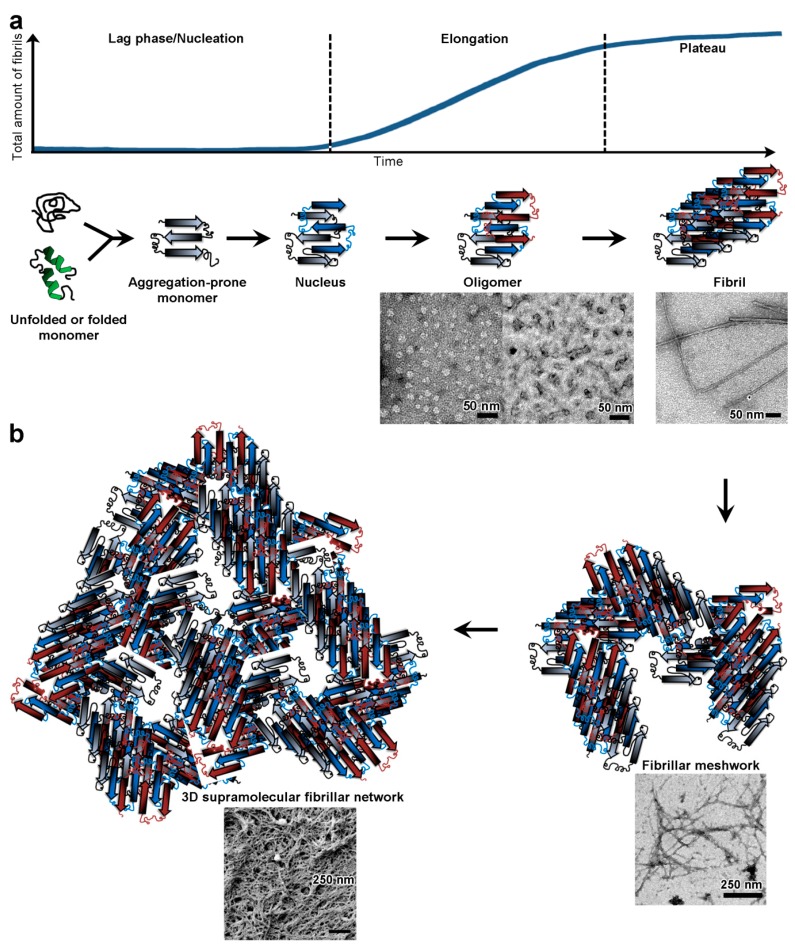
Figure 1.
From monomer to hydrogel. (a) Formation of amyloid fibrils. Amyloidogenesis is a nucleation-dependent polymerisation process, which shows a typical sigmoidal behaviour. When followed over time, fibril formation can classically be divided into three phases: nucleation typically characterised by a lag phase, elongation and a plateau. During nucleation, monomers (either unfolded or folded) have to undergo a conformational change to adopt an aggregation prone β-sheet conformation. Then aggregation-prone monomers have to come together in the right conformation and orientation, in an energetically unfavourable step, to form the minimal stable assembly, the nucleus. Once formed, the nucleus serves as a structural template for cooperative elongation. The assembly process becomes energetically favourable and proceeds through addition of aggregation-prone monomers onto the nucleus during elongation to form assembly intermediates or oligomers. Morphologically, by transmission electron microscopy, these oligomers appear as spherical structures (doughnut-like of 10–20 nm diameter) or small rods/protofibrils of various length (~20 to 70 nm). Oligomers carry on growing at the expense of monomers until the monomer concentration falls to the critical fibrillar concentration (the minimum monomer concentration required to form fibrils) and then fibril extension ceases (plateau phase). Typically, by transmission electron microscopy, fibrils can be several μm long with a width of 10 to 20 nm; (b) Formation of a 3D supramolecular fibrillar network. Beyond fibril formation, amyloid fibrils can interact with one another through a range of non-covalent and non-specific interactions to first form a fibrillar meshwork. By transmission electron microscopy, several μm long fibrils are seen to mostly laterally pack together, as well as twisting around one another. This fibrillar meshwork then proceeds, through further fibrillar interactions and entanglements, to form a 3D supramolecular fibrillar network. By scanning electron microscopy, the 3D network comprises fibril bundles as well as supramolecular networks of condensed amyloid fibrils. In an aqueous environment, this 3D supramolecular fibrillar network would be water-filled and act as the basis for hydrogel formation. This water-filled network has pore size defined by the fibrillar species and cross-linkers if present (see holes in between the schematic entangled β-sheets or within the condensed fibril in the scanning electron microscopy).