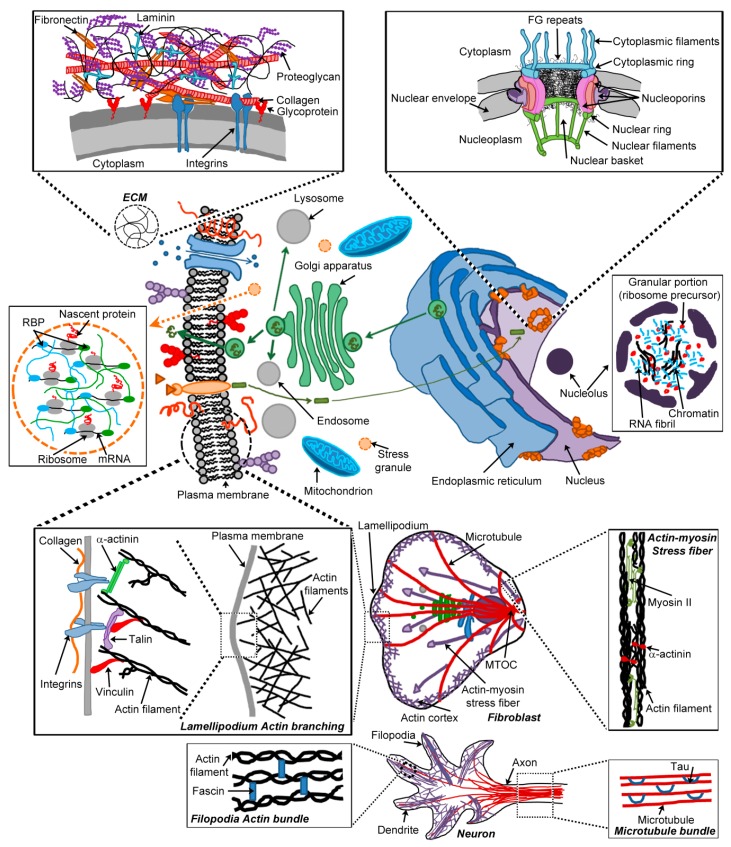
Figure 2.
Cellular hydrogels. Hydrogel-forming polypeptides can be found either within the eukaryotic cell (e.g., membrane-less organelles such as the nucleolus and stress granules, the central channel of the nuclear pore complexes (NPC), and the cytoskeleton) or in the extracellular space (extracellular matrix (ECM)). Hydrogel-forming polypeptides provide a wide range of functions for eukaryotic cells: selective diffusion barriers (ECM and NPC), compartmentalisation (nucleolus and stress granules), physical integrity (NPC, ECM and cytoskeleton), and motility (ECM and cytoskeleton). Some cellular hydrogels are formed by ‘functional’ amyloid-forming polypeptides (e.g., the central channel of the NPC and stress granules), but others (nucleolus, ECM and cytoskeleton) derive from non-amyloid polypeptides. For each cellular hydrogel, the hydrogel-forming polypeptide, cross-linkers and any other molecules involved in hydrogelation are depicted. Molecules that are involved in triggering polymerisation and/or polymerisation control, but not in hydrogelation, have been omitted. At the centre of the figure is a schematic of a typical eukaryotic cell, showing organelles and vesicular transport (green circles, containing proteins as green ‘lines’) between organelles of the endomembrane system: endoplasmic reticulum (light blue), Golgi apparatus (green), endosome (grey), lysosome (grey) and plasma membrane. Molecules present at the plasma membrane are also depicted: proteins (red ‘lines’) and glycoproteins (red lines with circles); proteoglycans (purple circles); receptors (light orange), their ligands (dark orange triangles) and downstream effectors (green rectangle); and transmembrane channel (blue) with molecules able to cross through it (blue circles). The ECM (top left inset) comprises proteoglycans and fibrous proteins such as collagen (red ‘tubes’), with the precise composition and organisation varying between tissue types. Collagen provides a structural framework for the ECM. Other proteins, such as fibronectin (orange) and laminin (blue), cross-link the ECM itself, but also the ECM to cells (via integrins, blue), and the ECM to soluble molecules. Proteoglycans (black fibrils with purple glycans) form the hydrogel, in which collagen and cross-linkers are embedded. NPC (top right inset) are spanning the nuclear envelope and formed from different protein types: filaments and rings (blue and green) forming the cytoplasmic and nucleoplasmic sides, and nucleoporins (purple, orange and pink) spanning the envelope. NPC selectively gate transport between the cytoplasm and nucleoplasm, which is mediated by a subclass of nucleoporins containing multiple Phenylalanine-Glycine (FG) repeats (FG-Nups). FG-Nups form an extended meshwork of fibrils (black filaments) lining the central channel and proposed to form a hydrogel with selective permeability. Stress granules (middle left inset) are membrane-less organelles accumulating during translational response to stress. They contain mRNA (black), translation machinery (e.g., ribosomes, grey) and RNA-binding proteins (RBPs; blue and green ovals). RBPs, through their prion-like domains, promote reversible aggregation, liquid-liquid phase separation followed by hydrogelation, which triggers formation of mature stress granules. The nucleolus (middle right inset) is also a membrane-less organelle maintained by aggregation, phase separation and hydrogelation. It is organised into three ‘compartments’: the granular portion (ribosome precursors, red), the fibrillar centre (RNA fibrils, blue) and the dense fibrillar portion (chromatin, black). The bottom third of the figure represents two types of cells (fibroblast and neuron), with different types of cytoskeleton organisation detailed (actin, purple, and microtubule, red). Cytoskeleton filaments form hydrogels cross-linked by a range of cytoskeleton-binding proteins. Just beneath the plasma membrane of some resting cells there is a cortex rich in actin. In eukaryotic cells, environment sensing and motility are mostly achieved through two types of protrusions, lamellipodium and filipodia, both formed via actin polymerisation generating treadmilling and driving directional movement at the cell leading edge. In lamellipodium (bottom left penultimate inset), actin polymerisation forms a dense network running in a crisscross fashion at angles of ~70°, crosslinked together by filamin (not shown). Directional migration is initiated by extracellular cues such as ECM proteins (e.g., collagen, orange filament). Protrusions are stabilised by adhesions linking the actin cytoskeleton to the underlying ECM proteins. In focal adhesions, integrins (heterodimeric receptor, blue) span the membrane and interact with the ECM substrate and, via actin-binding proteins (α-actinin, green, vinculin, red, and talin, purple), with intracellular actin. Filopodia are long thin protrusions composed of parallel polymerised actin bundles held together by a variety of proteins (e.g., fascin, blue) (bottom left inset). Attachment to the ECM substrate is followed by a contraction phase, detachment at the cell rear and retraction. Retraction requires a motor protein, myosin II (green), found in actomyosin stress fibers, crosslinked by α-actinin (red) (bottom right penultimate inset). Activation of the myosin motor leads to shortening of the filaments and subsequent cellular movements, but also promotes disassembly of adhesions at the cell rear. Microtubules radiate from the microtubule organising centre (MTOC) and are involved in moving and redistributing components of the cell. In neurons, reversible microtubule polymerisation in bundle is controlled by tau (blue semi-circle) binding (bottom right inset).