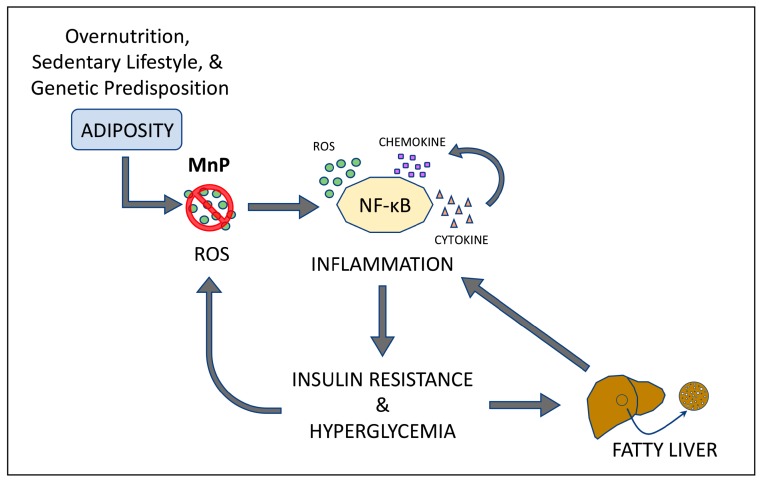
Figure 9.
Mechanism of redox modulation by MnP in a HFD model of obesity and insulin resistance. Increased caloric intake, coupled with decreased physical activity along with a genetic predisposition can lead to reactive oxygen species (ROS) production and oxidative stress [2,3,9,10,11]. Uncontrolled obesity-induced oxidative stress results in chronic inflammation and production and circulation of NF-κB-dependent proinflammatory cytokines and chemokines, as well as additional production of ROS [13,14,15], fueling a feedback loop. Inflammation can directly affect insulin signaling, resulting in insulin resistance and hyperglycemia. This not only contributes to the progression of type 2 diabetes (T2D), but also exacerbates ROS production and oxidative stress. Consequently, these events cause hepatic steatosis and subsequent non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of metabolic syndrome. In addition, there is a perpetuation of insulin resistance, as well as dyslipidemia—hallmarks of T2D progression. Treatment with MnP is known to not only scavenge superoxide, but also possess anti-inflammatory properties [19,21,22,23,24,27,28,29,37,38]. Blockade of nuclear NF-κB activation by MnP was sufficient to diminish pro-inflammatory cytokine production, thereby reducing weight gain, insulin resistance, and hepatic steatosis in HFD-fed mice.