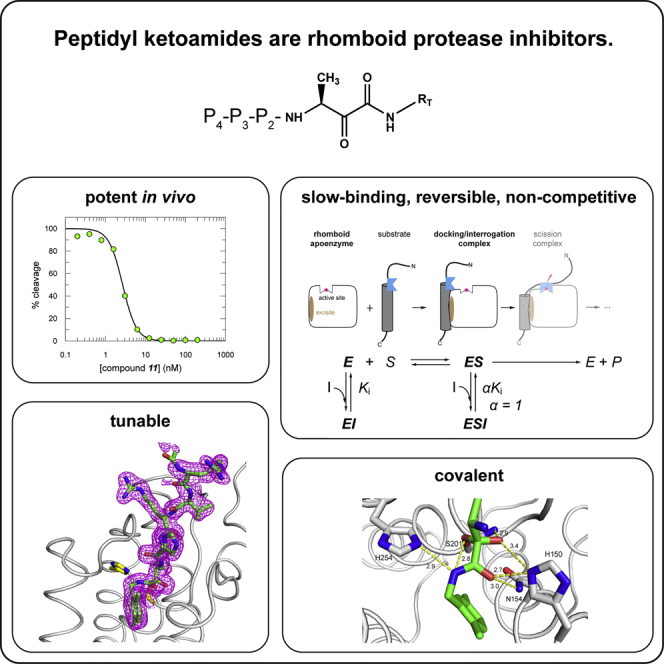
Summary
Rhomboid-family intramembrane proteases regulate important biological processes and have been associated with malaria, cancer, and Parkinson's disease. However, due to the lack of potent, selective, and pharmacologically compliant inhibitors, the wide therapeutic potential of rhomboids is currently untapped. Here, we bridge this gap by discovering that peptidyl α-ketoamides substituted at the ketoamide nitrogen by hydrophobic groups are potent rhomboid inhibitors active in the nanomolar range, surpassing the currently used rhomboid inhibitors by up to three orders of magnitude. Such peptidyl ketoamides show selectivity for rhomboids, leaving most human serine hydrolases unaffected. Crystal structures show that these compounds bind the active site of rhomboid covalently and in a substrate-like manner, and kinetic analysis reveals their reversible, slow-binding, non-competitive mechanism. Since ketoamides are clinically used pharmacophores, our findings uncover a straightforward modular way for the design of specific inhibitors of rhomboid proteases, which can be widely applicable in cell biology and drug discovery.
Keywords: intramembrane protease, rhomboid protease, mechanism, specificity, inhibition, inhibitor, ketoamide, crystal structure
Graphical Abstract
Highlights
-
•
N-substituted peptidyl α-ketoamides are nanomolar inhibitors of rhomboid proteases
-
•
Peptidyl ketoamides inhibit rhomboids covalently, reversibly, and non-competitively
-
•
The peptide and ketoamide substituent independently modulate potency and selectivity
-
•
Peptidyl ketoamides are selective for rhomboids, sparing most human serine proteases
Ticha et al. discover rhomboid protease inhibitors that are unprecedentedly potent and selective. They are based on a pharmacologically compliant chemotype and can thus serve as hitherto unavailable specific tools for cell biology or can yield lead compounds targeting rhomboids in medically relevant contexts such as malaria or Parkinson's disease.
Introduction
Rhomboid intramembrane proteases are evolutionarily conserved proteins with numerous important biological functions, including growth factor secretion, regulation of mitochondrial dynamics, and membrane protein quality control (Fleig et al., 2012). As such, they are being increasingly explored as potential drug targets, for example, for malaria (Baker et al., 2006, Lin et al., 2013, O'Donnell et al., 2006), cancer (Song et al., 2015), Parkinson's disease (Meissner et al., 2015), and diabetes (reviewed in Chan and McQuibban, 2013). These efforts are, however, hindered by the lack of selective and potent rhomboid inhibitors that could be used for cell biological studies, validation of therapeutic potential of rhomboids, and as templates for drug development (Strisovsky, 2016a). As explained elsewhere in more detail (Strisovsky, 2016a), the currently used inhibitors of rhomboid proteases suffer from drawbacks, making them unsuitable for these purposes. Isocoumarins are highly reactive and lack selectivity (Harper et al., 1985, Powers et al., 2002, Powers et al., 1989), β-lactams have limited potency in vivo (half maximal inhibitory concentration [IC50] ∼5–10 μM) (Pierrat et al., 2011), and β-lactones are not very potent (apparent IC50 of ∼40 μM) (Wolf et al., 2013). Furthermore, no rational strategy for modulation of their selectivity exists for any of these inhibitor classes. Here, we address both of these bottlenecks.
The principles of the mechanism and specificity of a protease determine to a large extent the strategies for inhibitor development (Drag and Salvesen, 2010). Rhomboids are serine proteases with a Ser-His catalytic dyad (Wang et al., 2006), and they recognize their transmembrane substrates in a two-tier process. It is assumed that first a portion of the transmembrane domain of the substrate docks into an intramembrane interaction site of rhomboid within the plane of the lipid bilayer, upon which a linear segment of the substrate (possibly generated by local unfolding of the top of the substrate's transmembrane helix) interacts with the water-exposed active site (reviewed in Strisovsky, 2016a, Strisovsky, 2013). This “recognition motif” encompasses the P4 to P2′ (Schechter and Berger, 1967) residues of the substrate (Strisovsky et al., 2009), it largely determines the kcat of the reaction (Dickey et al., 2013), and thus modulates selectivity toward a given rhomboid protease (Ticha et al., 2017). Recent reports have shown that peptidyl chloromethylketones (Zoll et al., 2014) and peptidyl aldehydes (Cho et al., 2016) are weakly inhibiting rhomboid proteases at medium to high micromolar concentrations, but they lack selectivity and their potency is insufficient for use as research tools.
Inspired by the current knowledge of the rhomboid protease mechanism (reviewed in Strisovsky, 2016a), we set out to explore the chemical space of oligopeptides equipped with electrophilic warheads in search of new rhomboid inhibitors of greater potency. Our systematic analysis resulted in the discovery of a modular scaffold based on peptidyl-ketoamide substituted with hydrophobic groups that represents a novel class of potent and selective rhomboid inhibitors. The in vivo activity of these compounds is in the low nanomolar range, which is up to three orders of magnitude more potent than any other currently known rhomboid inhibitors. Furthermore, we gained insight into the mode of binding of peptidyl ketoamides by solving their co-crystal structures with rhomboid protease, and we present strategies to modify their selectivity and potency on a systematic basis. We expect this compound class to find a widespread use in cell biology in rhomboid protease related contexts and to provide templates for the development of drugs targeting rhomboid proteases.
Results
The Potency of Substrate-Derived Peptidyl Chloromethylketone Inhibitors Can be Markedly Enhanced by Optimizing the Amino Acid Sequence of the P5 to P1 Region
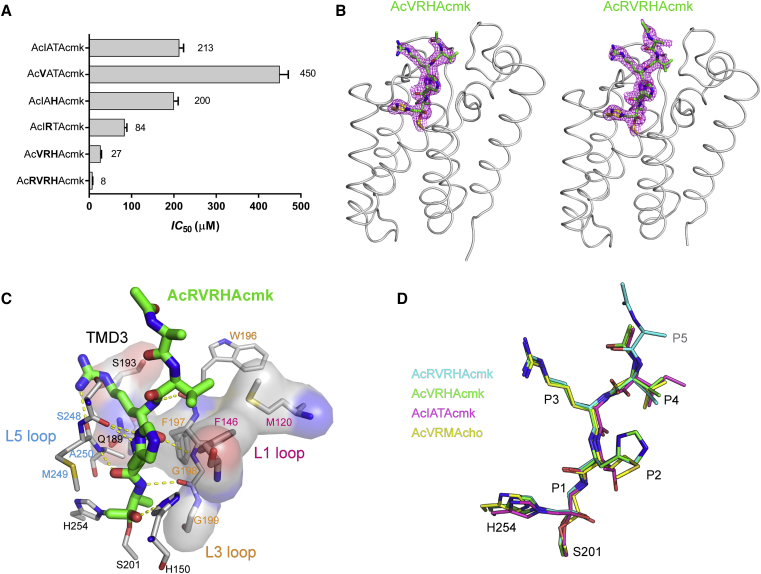
Rhomboid proteases exhibit discernible sequence preferences in the P5 to P2′ region of their substrates (Strisovsky et al., 2009, Zoll et al., 2014). To gain insight into these preferences and their possible interactions, we have generated tetra- and pentapeptidyl chloromethylketones (CMK or cmk henceforth) harboring amino acids preferred in positions P5 to P1 by the Escherichia coli rhomboid GlpG (GlpG henceforth), using the sequence background of the Providencia TatA (Stevenson et al., 2007), represented by the parent compound Ac-IATA-cmk. We measured the inhibitory properties of this series of compounds using a newly developed in vitro assay employing a fluorogenic transmembrane peptide substrate (Ticha et al., 2017) that faithfully represents a native rhomboid substrate. The effects of the mutations were additive, and the inhibitor containing the most favored amino acids in positions P5 to P1 (Ac-RVRHA-cmk) is approximately 26-fold more potent than the parent compound Ac-IATA-cmk (Figure 1A).
Figure 1.
The Potency of Substrate-Derived Inhibitors Can be Improved by Modifying the Amino acid Sequence of the P5 to P1 Region
(A) The parent inhibitor Ac-IATA-cmk was modified by introducing strongly preferred amino acids (Zoll et al., 2014) into the P4, P3, P2, and P5 positions to yield the listed compounds. Their apparent IC50 values were measured with 1 hr preincubation using 10 μM fluorogenic substrate KSp35 and 0.05% (w/v) DDM. The reported values are best-fit means with SD representative of 2–3 measurements.
(B) The sequence-optimized peptidyl chloromethylketones were soaked into the native crystals of GlpG and structures of the complexes were solved by X-ray diffraction (for statistics, see Table S1). In the displayed structures, the catalytic dyad is shown as yellow sticks and the inhibitors are shown as green sticks surrounded by the 2mFo − DFc electron density map contoured at 1σ and shown 1.6 Å around the stick model. Note that in the Ac-RVRHA-cmk structure (right), the side chain of the Arg residue in the P5 position of the inhibitor has not been modeled due to poor or missing electron density peaks.
(C) Interactions of RVRHA-cmk with GlpG were analyzed by Ligplot+ (Laskowski and Swindells, 2011). Ligands are shown as thick sticks with carbons in green, proteins as thin sticks with carbons in gray, hydrogen bonds as yellow dashed lines, and amino acids involved in van der Waals contacts are highlighted as transparent surfaces. The inhibitor forms covalent bonds with S201 and H254 via the chloromethylketone warhead, and it hydrogen bonds with the backbone of residues 196–198 from the L3 loop and residues 248–250 from the L5 loop. van der Waals contacts with the inhibitor are formed by the residues from the L3 loop of GlpG, by S193 and Q189 from TMD3, and by F146 and M120 from the L1 loop that pack against the Val side chain of the P4 position of the inhibitor, as observed previously (Zoll et al., 2014).
(D) The conformations of peptide inhibitors bound to the active site of GlpG were compared by performing structural alignment of the complexes of VRHAcmk (PDB: 5MT7), RVRHAcmk (PDB: 5MT8), IATAcmk (PDB: 4QO2), and VRMAcho (PDB: 5F5B) in PyMOL (Schrodinger, 2012). Note that the structure of RVRHAcmk suggests where the P5 amino acid points, but the density for this side chain is not visible beyond its β-carbon. The backbone of the ligands in all complexes has virtually identical conformation with the exception of the distortion of the oxyanion by the chloromethylketone, and the biggest differences are found in the conformation of the P2 side chain, which is not surprising, because almost any side chain can be accommodated in this position (Zoll et al., 2014).
To provide mechanistic explanation for the observed increase in inhibitory potency, we determined the structures of GlpG in complex with Ac-RVRHA-cmk and Ac-VRHA-cmk (Figure 1B). The side chain of Arg in the P5 position of Ac-RVRHA-cmk could not be modeled due to poor electron density, and the two structures are otherwise virtually identical; superposition of all corresponding Cα atoms yields a root-mean-square deviation of 0.19 Å per atom (using the SSM method as implemented in CCP4MG v2.10.4; Krissinel and Henrick, 2004, Mitchell et al., 1990). Both inhibitors interact with the L3 and L5 loops via main-chain hydrogen bonds, and via hydrogen bonds involving the side chains of the strongly preferred Arg and His in the P3 and P2 position, respectively (Figure 1C). These interactions are not observed in the structure of the parent compound Ac-IATA-cmk (Zoll et al., 2014), suggesting that they contribute to the higher potency of Ac-RVRHA-cmk and Ac-VRHA-cmk over Ac-IATA-cmk. The interactions of the residues in the P4, P3, and P2 positions with the enzyme are structurally independent, explaining why the effects of substitutions in these positions are additive (Figure 1A). The overall binding mode of both compounds into the rhomboid active site is similar to the binding mode of peptide aldehyde Ac-VRMA-cho (Cho et al., 2016) (Figure 1D). Collectively, these data show that rhomboid subsite preferences are additive in the context of an active site targeted inhibitor and that sequence optimization in this region can significantly increase the inhibitory potency of the compounds.
A Screen of Covalent Reversible Warheads for Inhibition of Rhomboid
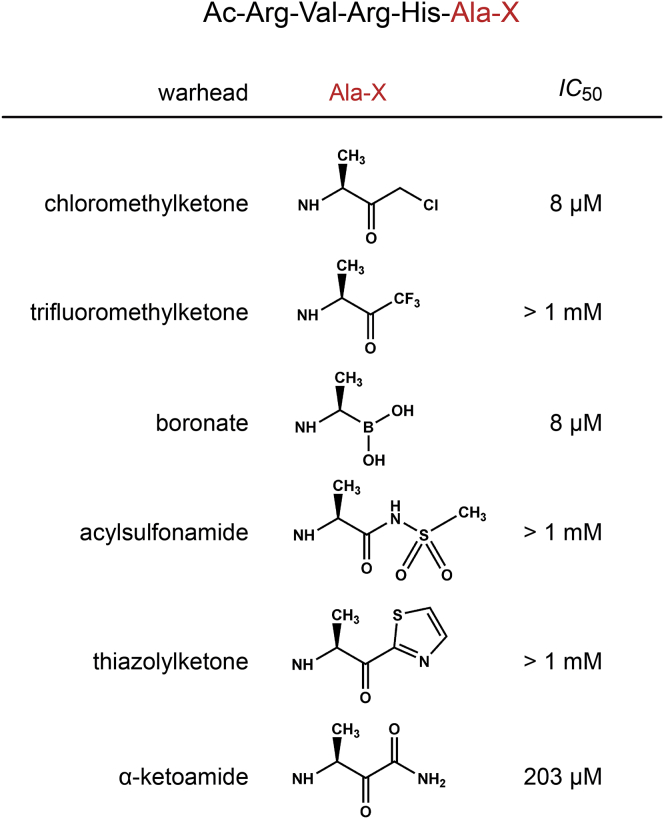
Since the sequence-optimized chloromethylketones are poor inhibitors with low micromolar IC50, we searched for alternative, more suitable electrophilic warheads that might improve the inhibitory potency. Furthermore, we reasoned that extending the inhibitor to the prime side of the active site might offer additional binding energy. We therefore synthesized a series of compounds based on the Ac-RVRHA sequence equipped with a selection of electrophilic, reversibly binding warheads commonly used for serine proteases in pharmacological settings (reviewed in Hedstrom, 2002, Walker and Lynas, 2001), including trifluoromethylketones, boronates, acylsulfonamides, thiazolylketones, and ketoamides (Figure 2), the last three of which can be extended into the prime side. We measured the apparent IC50 values of these compounds, and while trifluoromethylketones, acylsulfonamides, and thiazolylketones showed none or very weak inhibition in the millimolar range, the apparent IC50 of the boronate was 8 μM and of the ketoamide 203 μM under identical reaction conditions (Figure 2). Although the peptidyl boronate was the best of the series, it was still a relatively weak inhibitor comparable with the parent chloromethylketone, and it was not clear how to further improve its potency. The ketoamide was about 25-fold less potent, but since it could be extended to the prime side by a modification at the ketoamide nitrogen (Chatterjee et al., 1999, Liu et al., 2004), we next focused our attention on this class of compounds.
Figure 2.
A Screen of Electrophilic Warheads for the Inhibition of Rhomboid Proteases
The optimized parent sequence Ac-RVRHA was linked to electrophilic warheads commonly used for targeting serine proteases (reviewed in Hedstrom, 2002, Walker and Lynas, 2001). The apparent IC50 values of the compounds were measured in 0.05% DDM using 10 μM substrate KSp35 (Ticha et al., 2017) with 1 hr preincubation. Given are the mean values of 2–3 measurements.
Extensions at the Prime Side of Peptidyl Ketoamides Greatly Enhance Their Inhibitory Potency
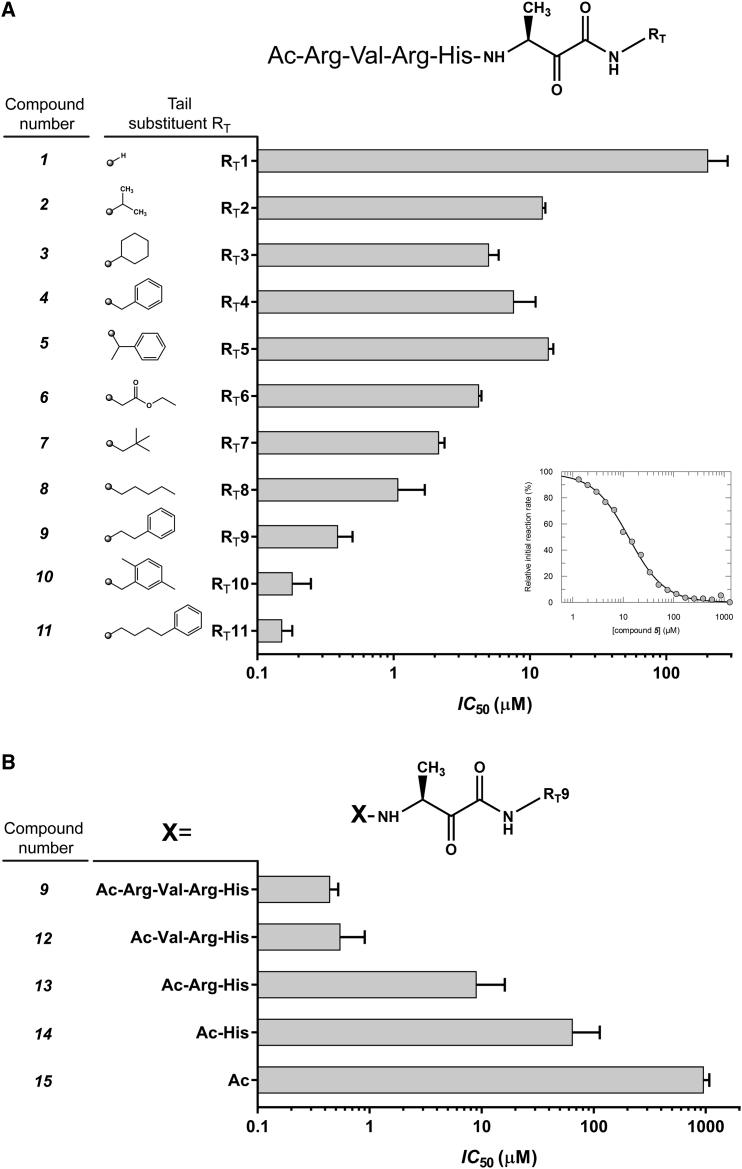
We hypothesized that extending the peptidyl ketoamides to the prime side of the active site might increase their potency, since the P2′ residue (hydrophobic in case of GlpG) was shown to be important for substrate recognition by rhomboids (Dickey et al., 2013, Strisovsky et al., 2009), and interactions of the substrate transmembrane domain beyond P2′ potentiate substrate cleavage in a detergent micelle assay (Ticha et al., 2017). We synthesized a series of peptidyl ketoamides based on the Ac-RVRHA sequence, bearing a mostly hydrophobic “tail” of increasing size at the ketoamide nitrogen (Figure 3A) that could reach far into the prime side of the rhomboid active site. The tail substituent indeed had a dramatic effect on the potency of the inhibitors in vitro (Figure 3A). The most effective compound of the series, bearing a 4-phenyl-butyl tail (compound 11), already displayed about 1,000-fold lower IC50 than the parent compound 1. The IC50 of 11 reaches half of the enzyme concentration used in the assay, suggesting that 11 is a potent inhibitor of GlpG.
Figure 3.
Modification of the Prime-Side Substituent at the Amide Group of Peptidyl Ketoamides Enhances Their Potency by Orders of Magnitude
(A) A screen of the effect of the tail substituent RT on the inhibitory properties of ketoamide inhibitors of GlpG based on the parent compound Ac-RVRHA-CONH2. The apparent IC50 values of all compounds were measured in 0.05% (w/v) DDM and 10 μM KSp35 (Ticha et al., 2017) with 1 hr preincubation. The IC50 values of the most effective compounds 9, 10, and 11 are three orders of magnitude lower than that of the parent compound 1. The reported values are best-fit means with SD representative of 2–3 measurements. The inset shows a typical inhibition curve.
(B) The significance of the peptidyl part in compound 9. The peptidyl part of 9 was progressively truncated from the N terminus, and the apparent IC50 values of all compounds were measured in 0.05% (w/v) DDM and 10 μM KSp35 (Ticha et al., 2017) with 1 hr preincubation. The reported values are best-fit means with SD representative of 2–3 measurements.
Next, we examined the relative importance of the peptidyl part for the inhibitory potency. We generated a series of progressively N-terminally truncated variants of 9 and measured their inhibitory potency against GlpG (Figure 3B). Removing the P5 Arg from 9 to yield 12 had virtually no effect on IC50 (0.44 versus 0.55 μM), while removing the P5 and P4 residues in 13 led to a ∼20-fold decrease in potency in comparison with the parent compound 9 (IC50 changes from 0.44 to 9 μM). Removing three residues (from P5 to P3) in 14 led to a dramatic ∼150-fold loss of potency, yielding a weak inhibitor with about 65 μM IC50, and the absence of the P5 to P2 residues in 15 resulted in a total ∼2,250-fold reduction in potency compared with 9 and IC50 higher than 1 mM. This experiment demonstrates that the non-prime (P4 to P1) and prime sides of the inhibitor contribute to its potency almost equally. The P5 residue can be omitted with only a marginal effect on inhibitory potency, which can be probably compensated by a suitable prime side tail substituent.
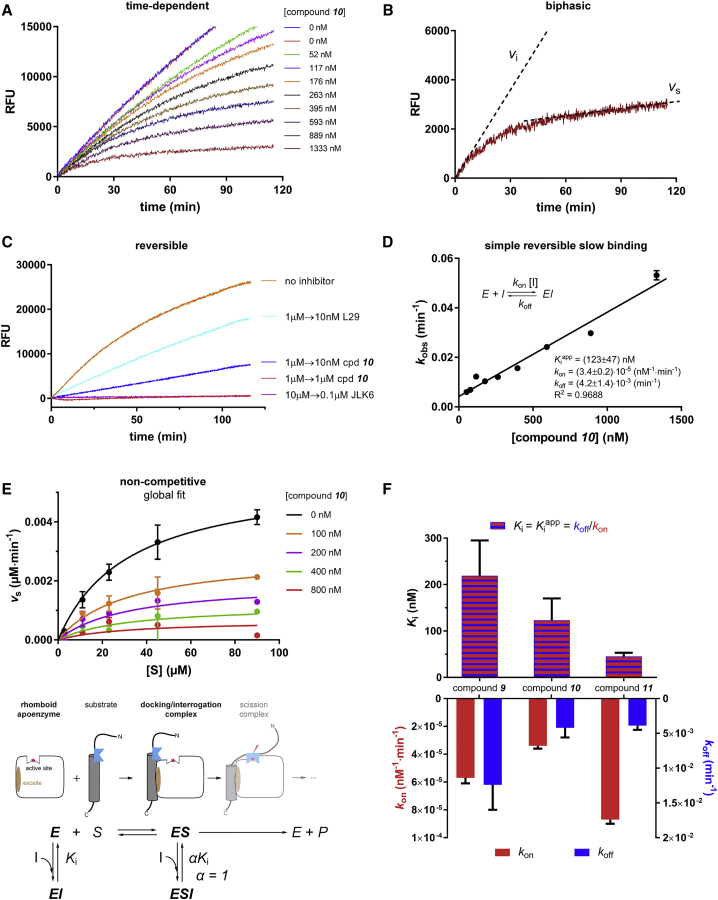
Ketoamides are known to be covalent reversible inhibitors of soluble serine proteases with a classical catalytic triad (Liu et al., 2004). Since rhomboids are unusual serine proteases using only a Ser-His dyad for catalysis (Wang et al., 2006), we investigated the mechanism of rhomboid inhibition by these compounds more closely. Progress curves measured at varying inhibitor concentrations (Figure 4A) had biphasic character; especially at the highest inhibitor concentrations tested, the reaction rate decreased over approximately the first hour and became more or less constant over the next hour (Figures 4A and 4B). This indicates that inhibition was time dependent, which is typical for slow-binding inhibitors (Copeland, 2013b). In addition, upon rapid dilution of inhibitor-saturated enzyme to a subinhibitory concentration, the reaction rate was partially recovered (Figure 4C), together indicating that peptidyl ketoamides exhibit slow-binding reversible behavior (Copeland, 2013a, Singh et al., 2011).
Figure 4.
Mechanism of Inhibition of Rhomboid Protease GlpG by Peptidyl Ketoamides Analyzed Using Fluorogenic Transmembrane Peptide Substrates
(A) Progress curves in the presence of increasing concentrations of compound 10 show biphasic character, which is typical for slow-binding inhibitors (Copeland, 2013a, Morrison, 1982). GlpG (0.5 nM) was incubated with 25 μM substrate KSp93 in the presence of 0.05% (w/v) DDM and 0–1,333 nM 10. Fluorescence at 493 nm was followed to monitor substrate cleavage.
(B) Biphasic progress curves characterized by an initial reaction rate (vi) and steady state reaction rate (vs). The progress curve at 1,333 nM compound 10 from the experiment in (A) is shown in detail, and both reaction rates obtained from non-linear regression into Equation (1) are shown as dotted lines.
(C) Reversibility of inhibition by ketoamides was assessed by the rapid dilution method (Harper et al., 1985, Harper and Powers, 1985). Compound 10 (1 μM) was pre-incubated with 0.4 μM GlpG, 0.05% (w/v) DDM at 37°C for 1 hr, leading to complete inhibition. This solution was then rapidly diluted 100-fold either into the reaction buffer containing 10 μM substrate KSp64 (Ticha et al., 2017) (yielding final 10 nM inhibitor) or into the reaction buffer with 10 μM substrate KSp64 and 1,000 nM 10. For comparison, we used β-lactam L29 (Pierrat et al., 2011) at 1 μM and isocoumarin JLK6 (Vinothkumar et al., 2010) at 10 μM as known reversible and irreversible inhibitors of rhomboid proteases, respectively. Activity recovery was followed by measuring fluorescence over the course of 120 min with excitation at 553 nm and emission at 583 nm.
(D) Progress curves of KSp93 cleavage at increasing concentrations of 10 measured under (A) were analyzed by non-linear regression as described for slow-binding inhibition (Copeland, 2013a, Morrison, 1982) using GraphPad Prism version 7.02 for Windows (GraphPad Software, La Jolla, California, USA) to yield the rate constant for the onset of inhibition, kobs. The linear character of the dependence of kobs on inhibitor concentration is typical for a simple slow-binding mechanism (inset), and its linear regression allows determination of the underlying apparent inhibitory constant and its constituent rate constants kon and koff (inset). The kobs values are reported as best-fit mean ± SD.
(E) The influence of inhibitor concentration on the apparent KM and kcat suggests the mode of inhibition by compound 10. Michaelis curves at the indicated inhibitor concentrations were measured by plotting vS (measured as in Figure 4B) against substrate concentration using 1 nM GlpG, 0.15% (w/v) DDM and highly sensitive substrate KSp96. The data were globally fitted to the models of competitive, non-competitive (figure top), uncompetitive, and mixed inhibition as implemented in GraphPad Prism 7.02, and their statistical analysis yielded the non-competitive mechanism (figure bottom) as the best fit. The middle of the figure shows a schematic mapping of this mechanism onto the consensual model of substrate recognition by rhomboid proteases. The data points in the Michaelis plots (figure top) represent means ± SD of duplicate measurements.
(F) Summary of inhibition kinetics parameters of compounds 9, 10, and 11. The apparent inhibitory constants (blue-red striped columns) and the constituent rate constants kon (red columns) and koff (blue columns) were determined from progress curve analysis as shown in (A and C) (note that = koff/kon). For non-competitive inhibitors, the true inhibitory constant Ki equals . Note that 11 is a highly potent inhibitor with Ki of (45 ± 8) nM. Graphs show best-fit means with SDs.
The slow-binding reversible inhibition mechanism can be formally divided into two steps. First, an initial encounter complex (EI) forms, and then a slow step leads to the much more stable EI* complex (E + I ↔ EI ↔ EI*), usually involving a significant conformational change of the enzyme (Copeland, 2013a). To analyze the contribution of each of these two steps to the mechanism of inhibition of rhomboids by peptidyl ketoamides, we investigated the concentration and time dependence of inhibition kinetics by 10. The “bending” of biphasic progress curves (Figures 4A and 4B) reflects the rate of “onset of inhibition” described by the rate constant kobs, which can be obtained from progress curve data using non-linear fitting to Equation 1:
| (Equation 1) |
where [P] is the concentration of the reaction products, vi is the initial reaction rate in the first phase of the biphasic progress curve, and vs is the steady-state reaction rate (Figure 4B). Analysis of progress curves from Figure 4A showed that vi was independent of inhibitor concentration, and the plot of kobs against inhibitor concentration fitted well to a linear dependence (Figure 4D). Both phenomena are typical for simple (single-step) slow-binding inhibition (E + I ↔ EI) (Figure 4D); in other words, peptidyl ketoamides behave as “regular” reversible inhibitors but with very low rate constants for association and dissociation (Copeland, 2013a, Morrison, 1982), leading to the slow-binding kinetics. The application of this model yields the apparent inhibitory constant (i.e., not taking into account the inhibition modality and the influence of the substrate) for 10 of (123 ± 47) nM (Figure 4D).
The true inhibitory constant Ki, which is an important, substrate-independent property of an inhibitor, can be calculated from the apparent inhibitory constant , depending on the inhibitory modality and kinetic parameters of the substrate used. Global non-linear regression fitting of Michaelis curves measured in the presence of increasing concentrations of 10 (plotting vs against [S]) shows that the experimental data are best described by a non-competitive inhibition model (Figure 4E). This inhibition mode means that the inhibitor can bind both to the free enzyme and to the enzyme-substrate complex; in this case specifically, the affinities of the inhibitor to both forms of the enzyme are equal (α = 1) (Copeland, 2013a). Although non-competitive modality is non-typical for slow-binding inhibitors, it is conceivable why it is plausible in the case of peptidyl ketoamides and rhomboids. Several studies have suggested that substrate recognition by rhomboid proteases proceeds in two steps, via a docking/interrogation complex, where only a part of substrate's transmembrane domain interacts with rhomboid, followed by the interaction of the recognition motif with the active site forming the scission-competent complex (Cho et al., 2016, Strisovsky, 2016a, Strisovsky, 2016b, Strisovsky et al., 2009) (Figure 4E). Since the active site is unoccupied in the docking complex, binding of an active site-directed inhibitor is possible (Figure 4E), resulting in non-competitive behavior. Under this mechanism of inhibition, the true Ki is identical to (Copeland, 2013a, Purich, 2010). Similar progress curve analyses of 9 and 11 yield their kon and koff rate constants, their , and the true Ki values of (219 ± 76) nM and (45 ± 8) nM, respectively (Figure 4F and Table 1). In summary, this kinetic analysis shows that the peptidyl ketoamides described here are high-affinity inhibitors of rhomboid proteases unprecedented in the literature.
Table 1.
Summary of the Inhibition Properties of Compounds 9–11
| Compound | GlpG |
YqgP |
|||
|---|---|---|---|---|---|
| Ki (nM) | kon (10−6 nM−1 ·min−1) | koff (10−3 min−1) | IC50In Vivo (nM) | IC50In Vivo (nM) | |
| 9 | 220 ± 80 | 5.7 ± 0.4 | 12.0 ± 0.4 | 8.8 ± 0.4 | ND |
| 10 | 120 ± 50 | 3.4 ± 0.2 | 4.2 ± 1.4 | 6.0 ± 0.1 | ND |
| 11 | 45 ± 8 | 8.7 ± 0.3 | 3.9 ± 0.6 | 2.7 ± 0.1 | ∼5–10 |
Values for GlpG are reported as means ± SD.
Selectivity of Peptidyl Ketoamides
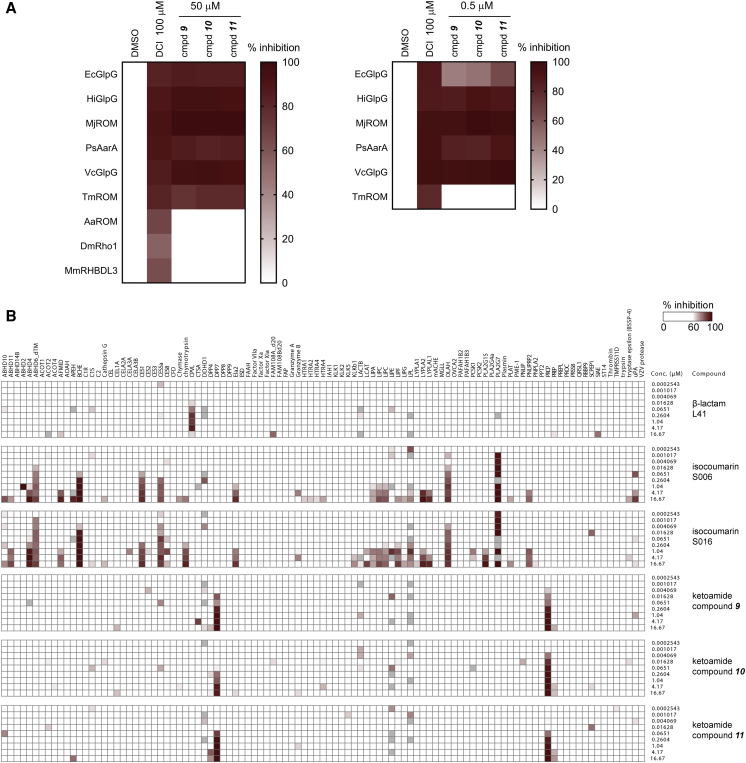
Any enzyme inhibitors to be used as specific tools for cell biology or as starting points for drug development must show sufficient level of selectivity toward their intended target. This is particularly important for compounds that react with the catalytic nucleophile common to many serine hydrolases. Only limited tests of selectivity have been conducted for the currently used rhomboid inhibitors isocoumarins, β-lactams and β-lactones, at best interrogating them against trypsin or chymotrypsin (Pierrat et al., 2011, Vosyka et al., 2013). To map the selectivity of peptidyl ketoamides more objectively and widely, we employed activity-based probe (ABP) competition assays that enable a more general and substrate-independent measurement of inhibitory potency, because they rely solely on the competition between a fluorescently labeled activity-based probe and the tested inhibitor (Nguyen et al., 2015, Serim et al., 2012). The assays we employed used fluorophosphonate ABPs that target the catalytic serine of a wide-range of serine hydrolases (Bachovchin et al., 2014), including rhomboids (Xue et al., 2012), and are thus very practical general detection reagents even for serine hydrolases for which sensitive substrates might not be available.
First, we tested 9, 10, and 11 against a panel of bacterial and eukaryotic rhomboid proteases (Wolf et al., 2015), and found that all three compounds potently competed with ABP labeling of rhomboids from bacterium Providencia stuartii (AarA), archaebacterium Methanocaldococcus jannaschii (MjROM), and three closely related rhomboids from bacteria E. coli (EcGlpG), Haemophilus influenzae (HiGlpG), and Vibrio cholerae (VcROM). Compounds 9, 10, and 11 outcompeted the ABP even at a concentration of 500 nM, suggesting that they were potent inhibitors of these rhomboid proteases. In contrast, none of these compounds were able to compete with the ABP labeling of rhomboid protease from the bacterium Aquifex aeolicus (AaROM), rhomboids from Drosophila (DmRho1) and mouse (MmRHBDL3), and they only partially inhibited labeling of rhomboid protease from bacterium Thermotoga maritima (TmROM) at 50 μM (Figure 5A). These data demonstrate that already these first-generation peptidyl ketoamides can discriminate between diverse rhomboid proteases.
Figure 5.
Selectivity of Peptidyl Ketoamides
(A) Selectivity of compounds 9, 10, and 11 for nine rhomboid proteases was profiled using activity-based probe competition assay at 50 μM and 0.5 μM concentration. The upper limit of enzyme concentration was 0.4 μM.
(B) Selectivity of compounds 9, 10, and 11 against human serine hydrolases was analyzed using EnPlex as described (Bachovchin et al., 2014).
We next examined peptidyl ketoamides for their possible off-target effects on other serine proteases. To get a representative picture of the selectivity of peptidyl ketoamides, we employed a recently developed EnPlex technology, which allows multiplex analysis of ABP competition with about 100 human serine hydrolases, mostly proteases (Bachovchin et al., 2014). Profiling of 9, 10, and 11 showed that in the concentration range where they inhibit rhomboid proteases, they fail to inhibit most of the tested human serine hydrolases with the exception of prolylcarboxypeptidase (PRCP) and the sequence related dipeptidylpeptidase 7 (DPP7) (Figure 5B). To put this into the context of the current generation of rhomboid inhibitors, isocoumarins S006 and S016 (Vosyka et al., 2013) hit about a dozen serine hydrolases in the same concentration range. The β-lactam L41 (Pierrat et al., 2011) inhibited appreciably only one enzyme (predicted serine carboxypeptidase CPVL), but it is much less potent on rhomboids than 9, 10, and 11, and it does not inhibit GlpG completely in vivo (Pierrat et al., 2011). The selectivity profile of ketoamide inhibitors of rhomboids is similar to the profile of clinically used ketoamide inhibitors of the hepatitis C protease (Bachovchin et al., 2014), indicating that the rhomboid-targeting N-modified peptidyl ketoamides are sufficiently selective with minimal risk of cross-reactivity against other serine proteases.
Peptidyl Ketoamides Potently Inhibit Rhomboids in Living Cells
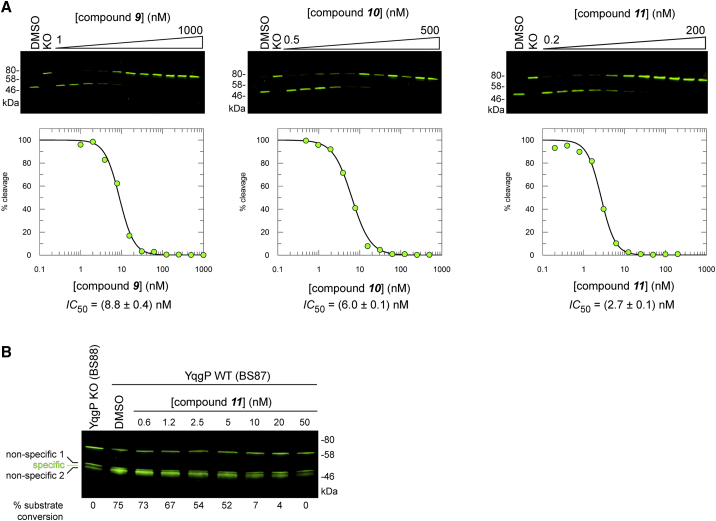
Having established the mechanism of rhomboid inhibition by peptidyl ketoamides in detergent micelles, and having shown that 9, 10, and 11 are able to inhibit potently rhomboid proteases from several Gram-negative bacteria (Figure 5A), we next tested whether the inhibitors will be able to target rhomboid proteases embedded in their native lipid bilayer in live cells. First, we expressed the model substrate derived from LacYTM2 in E. coli expressing endogenous levels of GlpG, incubated the bacterial cultures in the presence of increasing concentrations of 9, 10, and 11, and detected the steady-state levels of substrate processing by quantitative near-infrared western blotting (Figure 6A). The calculated substrate conversion values relative to the uninhibited reaction were plotted against the inhibitor concentration yielding the in vivo IC50 values. Strikingly, the most effective compound 11 had an in vivo IC50 value of 2.7 nM, which is three orders of magnitude lower than any other currently known rhomboid inhibitors (Cho et al., 2016, Pierrat et al., 2011).
Figure 6.
Peptidyl Ketoamides Potently Inhibit Rhomboid Activity in the Membranes of Living Cells
(A) Inhibition of endogenous GlpG by compounds 9, 10, and 11 in the membranes of live E. coli. The substrate MBP-FLAG-LacYTM2-Trx (Strisovsky et al., 2009) was expressed in wild-type E. coli NR698 with genetically permeabilized outer membrane (Ruiz et al., 2005) in the presence of increasing concentrations of inhibitors as described in STAR Methods. Substrate cleavage was measured in cell lysates by immunoblotting for FLAG and quantified using near-infrared fluorescence. The reported in vivo IC50 values are best-fit means with SD representative of 2–3 measurements. DMSO, dimethylsulfoxide vehicle control; KO, E. coli glpG::tet.
(B) Inhibition of endogenous YqgP by compound 11 in the membranes of live B. subtilis. The substrate AmyESP-MBP-FLAG-LacYTM2-Trx-HA was expressed in Bacillus subtilis 168 (ydcA::neo, xdkE::AmyESP-MBP-LacYTM2-Trx(erm, lin)) (BS87) in the presence of increasing concentrations of inhibitors as described in STAR Methods. Substrate cleavage was detected in cell lysates by immunoblotting for FLAG and detection by near-infrared fluorescence. Unspecific cleavage of the substrate was corrected for by substracting the intensity of the unspecific bands formed in the YqgP knockout control cells (BS88) from the product band and the closely co-migrating unspecific bands observed in the YqgP positive cells (BS87). This treatment was necessary because the specific cleavage product could not be resolved sufficiently well from the non-specific bands to be integrated separately. DMSO, dimethylsulfoxide vehicle control; YqgP KO, Bacillus subtilis 168 (ydcA::neo, yqgP::tet, xdkE::AmyESP-MBP-LacYTM2-Trx(erm, lin)) (BS88).
We then extended the range of organisms to Bacillus subtilis, a representative of Gram-positive bacteria, which have a thick cell wall and include major pathogens such as Staphylococcus, Listeria, Streptococcus, and others. Since the endogenous substrate of the B. subtilis rhomboid protease YqgP is unknown, and no robust and rescuable phenotypes have been reported for YqgP, we focused on inhibition of cleavage of a model substrate. Of the common model rhomboid substrates, YqgP cleaves LacYTM2 reasonably well (Ticha et al., 2017). We have thus expressed MBP-LacYTM2-Trx (Strisovsky et al., 2009) from the ectopic xkdE locus (Gerwig et al., 2014) in the wild-type B. subtilis 168 (BS87) and its yqgP deletion mutant (BS88) on an otherwise rhomboid-free background. Although the substrate was to some extent truncated by unknown processes in the ΔyqgP strain, a specific, closely co-migrating rhomboid-generated N-terminal cleavage product (Figure 6B) was produced in the YqgP wild-type strain BS87 but not in the ΔyqgP strain BS88 (Figure 6B). In the absence of any inhibitors, MBP-LacYTM2-Trx was cleaved to about 75% conversion by the endogenous YqgP, and addition of 11 into the growth media completely inhibited substrate cleavage at 50 nM (Figure 6B), indicating that the compound can penetrate the Gram-positive cell wall easily. Moreover, since compound 11 also inhibits several homologs of GlpG (Figure 5A), it is safe to assume that YqgP orthologs in other Bacilli, Lactobacilli, Staphylococci, and Listeria might be equally susceptible to inhibition by the described inhibitors, and compound 11 and its analogs can be directly used for chemical proteomics and cell biological studies of rhomboid proteases in Gram-positive bacteria.
N-Modified Peptidyl Ketoamides Bind the Rhomboid Active Site in a Substrate-like Manner Occupying the S4 to S2′ Subsites
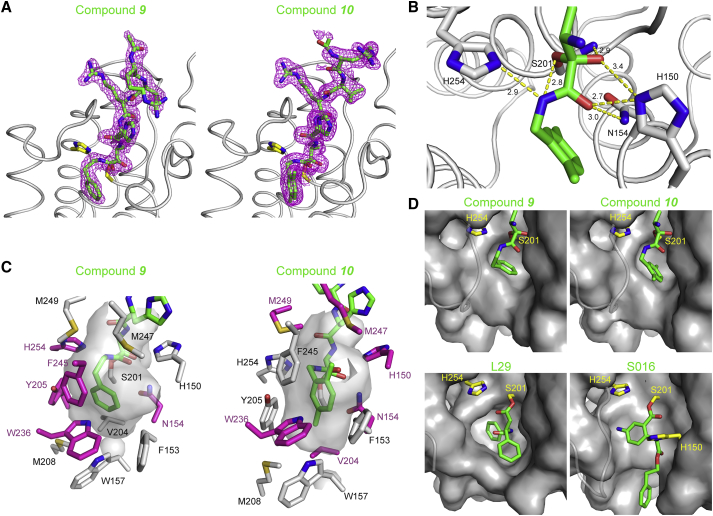
To understand why peptidyl ketoamides are such efficient rhomboid inhibitors and to establish the basis for structure-guided design of their improved variants, we determined the co-crystal structures of GlpG with 9 and 10 (Figure 7A). The complexes were formed by soaking the inhibitors into apoenzyme crystals, and the structures were solved using diffraction data to 2.16 and 1.78 Å resolution, respectively, allowing detailed comparison of their binding modes. In both cases, the pentapeptide RVRHA binds the active site cavity as an extended β strand, virtually identically to the binding mode of Ac-RVRHA-cmk (Figure 1C). We do observe electron density for the side chain of arginine in the P5 position in both structures, but its conformation differs between 9 and 10 (Figure 7A), and it is influenced by crystal contacts with the same residue from a neighboring molecule in the crystal (data not shown).
Figure 7.
N-Substituted Peptidyl Ketoamides Bind GlpG in a Substrate-like Manner and Occupy the S4 to S2′ Subsites of the Rhomboid Active Site
(A) Electron density map and ligand stick model of 9 and 10 in complex with GlpG. Compounds 9 and 10 were soaked into the native crystals of GlpG, and the structures of the complexes were solved by X-ray diffraction (for statistics, see Table S1). The catalytic dyad is shown as yellow sticks and the inhibitors as green sticks surrounded by the 2mFo − DFc electron density map contoured at 1σ and shown 1.6 Å around the inhibitor model. In the complex of 9, the electron density for the Arg residue in the P5 position was weaker, and the side chain has been modeled in a different conformation than in the complex of 10, which was solved to a higher resolution and where the side chain of the Arg in the P5 position is defined clearly.
(B) Hydrogen bond engagement by the warhead of compound 10 in the active site of GlpG was analyzed using the HBplus program (McDonald and Thornton, 1994) implemented in Ligplot+ (Laskowski and Swindells, 2011) with default criteria (donor … acceptor [D … A] distance cutoff of 3.9 Å; donor … acceptor-acceptor antecedent [D … A-AA] angle of 90°).
(C) Interaction pattern of inhibitor tails in the S2′ site of GlpG. The cavity surrounding the tails of 9 and 10 is shown as an inverse surface, and the side chains lining the cavity are shown as sticks. The residues engaged in van der Waals interactions (identified by Ligplot+) with the tails of the inhibitors are shown in magenta.
(D) Comparison of binding modes of the S2′ binding moieties in compounds 9, 10, L29 (Pierrat et al., 2011), and S016 (Vosyka et al., 2013) in the respective complex structures PDB: 5MT6, 5MTF, 3ZMI, and 3ZEB. Protein is showed as a gray surface, catalytic dyad carbons in yellow, and ligand carbons in green. The L5 loop residues 245–250 are shown as semitransparent loops for clarity. All structures are oriented in the same way.
In both inhibitors, the ketoamide warhead is covalently bonded via its proximal carbon to the side-chain oxygen of the catalytic S201, and it engages in a network of six hydrogen bonds in the active site (Figure 7B). The oxyanion formed by the proximal carbonyl oxygen accepts hydrogen bonds from His150 and the main-chain amide nitrogen of the catalytic serine, and the distal ketoamide carbonyl oxygen accepts hydrogen bonds from both H150 and N154, thus amply saturating the hydrogen-bonding groups engaged in the stabilization of the oxyanion (Cho et al., 2016). Furthermore, the ketoamide nitrogen donates a hydrogen bond to H254 and to the S201 side-chain oxygen covalently bound to the warhead. The resulting network of six hydrogen bonds (Figure 7B) probably helps position the ketoamide warhead in the proximity of the hydroxyl of the catalytic S201 to enhance its chemical reactivity in a conformation-dependent manner.
The tail substituents of 9 and 10 (RT9 and RT10) interact with the prime side of GlpG, buried in a cavity delimited by the side chains of amino acids F245, M247, and M249 from the L5 loop, W236 from TMD5, F153 and W157 from TMD2, and residues V204, M208, Y205, H254, H150, and N154 (Figure 7C). The different sets of residues making van der Waals contacts with each ketoamide tail are shown in magenta. The NH group of the side chain of W236 seems to form a weak H-π bond with the phenyl ring of the tail of both compounds, and F245 engages in π…π stacking against the dimethylbenzyl in RT10 (Figure 7C). Structural alignment of both ketoamide complexes to the complex of the β-lactam L29 (Vinothkumar et al., 2013) and isocoumarin S016 (Vosyka et al., 2013) (Figure 7D) shows that the tails of 9 and 10 bind in a similar area (the S2′ subsite) as the significantly larger groups of inhibitors L29 and S016. This alignment shows that the prime side of the GlpG active site is rather malleable, and larger or branched tails could be accommodated at the amide nitrogen of peptidyl ketoamides. This is likely to provide additional selectivity or binding energy and delineates one possible direction of further development of ketoamides as rhomboid inhibitors. The results presented here open the door to systematic development of rhomboid protease inhibitors in medically relevant contexts such as malaria (Baker et al., 2006, O'Donnell et al., 2006), Parkinson's disease (Chu, 2010, Meissner et al., 2015), and cancer (Song et al., 2015).
Discussion
Here, we discover that peptidyl ketoamides bearing a substantial hydrocarbon modification at the ketoamide nitrogen are efficient inhibitors of rhomboid intramembrane proteases, superior to any known rhomboid inhibitors in selectivity and by up to three orders of magnitude in potency. We also show that both of these properties are tunable by optimization of the peptide sequence and the character of the ketoamide “tail” substituent, defining a platform for the development of specific and potent rhomboid inhibitors. Since ketoamides are clinically used pharmacophores (Njoroge et al., 2008), our discovery of this pharmacologically compliant chemotype for rhomboid proteases enables the design of rhomboid inhibitors for cell biological and pharmacological use.
Structural analysis of peptidyl ketoamides complexed to GlpG reveals that they bind in a substrate-like manner, occupying the P4 to P2′ subsites (Figure 7A). The presence of residues in the P5 and P6 positions has been reported to improve the inhibition potency of peptidyl aldehydes significantly, but these residues could not be observed in any co-crystal structures (Cho et al., 2016). We do observe weak electron density for the side chain of Arg in the P5 position, but its conformation in the final crystallographic models of the complexes of 9 and 10 differs, indicating some degree of flexibility, and it is probably influenced by crystal contacts. In addition, the P5 residue does not contribute significantly to the inhibition potency of 9 (Figure 3B) and is thus dispensable.
The binding mode of peptidyl ketoamides suggests that they can access the rhomboid active site from bulk solvent, and probably do not need prior partitioning into the membrane. They are covalent (Figure 7) and reversible (Figure 4C), and their kinetics of binding to rhomboid is adequately described by a one-step slow-binding mechanism (Figure 4D). Their inhibition modality is non-competitive (Copeland, 2013a) (Figure 4E), implying that they can bind to the free enzyme as well as the docking/interrogation complex during rhomboid catalysis (Strisovsky, 2016a). This is consistent with the proposed mechanism of inhibition of rhomboid protease GlpG by peptidyl aldehydes (Cho et al., 2016).
For the development of peptidyl ketoamides as rhomboid inhibitors, subsite preferences of the given rhomboid protease must be mapped efficiently. This could be achieved using classical positional scanning peptide libraries starting from a known substrate sequence. Given that the effects of the amino acids in the P5–P1 positions are additive (Figure 1A), the optimal substrate could be formed by combining the single subsite preferences identified in the positional scan. An alternative method for mapping subsite preferences at both the prime and non-prime sides could be multiplex substrate profiling using designed peptide libraries and mass spectrometry (O'Donoghue et al., 2012), although its application to rhomboids has not been tested yet.
The second module determining the potency and selectivity is the tail substituent at the ketoamide nitrogen. Here, the effects of flexibility versus rigidity, branching, and polarity of the substituents need to be investigated to explore the available chemical and conformational space. A more speculative direction of further improvement of the inhibitors may involve cyclization via the tail substituent and the P2 residue, which seems sterically possible and unobstructive in the enzyme-inhibitor complex (Figure 7A). Such cyclization could improve the potency of the inhibitor by conformationally restricting it near the bound conformation.
Finally, peptidyl ketoamides have been used clinically to treat hepatitis C infection (boceprevir, telaprevir) (Njoroge et al., 2008), suggesting that both the intracellular availability and metabolic stability of rhomboid-targeting peptidyl ketoamides can most likely be modified for compliance with pharmacological needs. The potential of rhomboid inhibitors in pharmacologically relevant settings has yet to be proven, but it currently seems that inhibitors of Plasmodium rhomboids might be therapeutic for malaria (Baker et al., 2006, Lin et al., 2013), inhibitors of the human mitochondrial rhomboid protease PARL might stimulate mitophagy (Meissner et al., 2015) and thus be disease-modifying in the context of Parkinson's disease (Chan and McQuibban, 2013), and inhibitors of human RHBDL4 could be targeting EGF receptor signaling by transforming growth factor α in colorectal cancer (Song et al., 2015). Specific rhomboid protease inhibitors such as those that we describe here will serve as key tools for the validation and exploitation of these and other upcoming therapeutic opportunities involving rhomboid proteases.
Significance
Intramembrane proteases of the rhomboid family are widely conserved and have been implicated in malaria, colon cancer, and Parkinson's disease. They represent potentially attractive drug targets, but until now, no specific, potent, and pharmacologically compatible inhibitors have been available. Here, we discover that peptidyl ketoamides are the first such potent and specific inhibitors of rhomboid proteases, and we delineate a general modular way for their design against diverse rhomboid enzymes. This discovery can have a broad impact on the cell biology of rhomboid proteases and on drug discovery targeting this family of enzymes in the context of infectious diseases, cancer, and neurodegeneration.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-DYKDDDDK | Cell Signaling Technology | Cat#2368 |
| Monoclonal ANTI-FLAG® M2 antibody produced in mouse | Sigma | Cat#F1804 |
| Donkey anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, DyLight 800 | Invitrogen | Cat#SA5-10044 |
| Bacterial and Virus Strains | ||
| E. coli NR698 | Laboratory of Tom Silhavy (Princeton) | (Ruiz et al., 2005) |
| E. coli NR698ÄglpG::tet | Laboratory of Matthew Freeman (Oxford) | (Pierrat et al., 2011) |
| Bacillus subtilis 168 | Bacillus Genetic Stock Center | |
| Bacillus subtilis 168 ydcA::neo | This work | BS2 |
| Bacillus subtilis 168 ydcA::neo, yqgP::tet | This work | BS4 |
| Bacillus subtilis 168 ydcA::neo, xdkE::MBP-LacYTM2-Trx(erm, lin) | This work | BS87 |
| Bacillus subtilis 168 ydcA::neo, yqgP::tet, xdkE::MBP-LacYTM2-Trx(erm, lin) | This work | BS88 |
| E. coli C41(DE3) | Lucigen | Cat#60452-1 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| h-KRHDIN(E-edans)ISKSDTG(K-dabcyl)IFAAISLFSLLFQPLFGLSKK-nh2 | (Ticha et al., 2017) | KSp35 |
| h-KRHRI(E-edans)RVRHADTG(K-dabcyl)IFAAISLFSLLFQPLFGLSKKR-nh2 | This work | KSp93 |
| h-KRHRI(K-tamra)RVRHADTG(C-qxl610)IFAAISLFSLLFQPLFGLSKKR-nh2 | (Ticha et al., 2017) | KSp64 |
| h-KRHRINRVR(E-edans)ADTG(K-dabcyl)IFAAISLFSLLFQPLFGLSKKR-nh2 | This work | KSp96 |
| TAMRA-XP | Thermo Fisher Scientific | Cat#88318 |
| Revert Total Protein Stain Kit | LI-COR, Inc. | Cat#926-11010 |
| 3,4-dichloroisocoumarin (DCI) | Sigma | D7910 |
| AcRVRHAcmk | This paper | |
| AcVRHAcmk | This paper | |
| AcIATAcmk | Zoll et al., 2014 | |
| AcVATAcmk | This paper | |
| AcIAHAcmk | This paper | |
| AcIRTAcmk | This paper | |
| AcRVRHA-trifluoromethylketone | This paper | |
| AcRVRHA-boronate | This paper | |
| AcRVRHA-acylsulfonamide | This paper | |
| AcRVRHA-thiazolylketone | This paper | |
| AcRVRHA-CONH2 | This paper | |
| Ac-RVRHA-CONH-isopropyl | This paper | |
| Ac-RVRHA-CONH-cyclohexyl | This paper | |
| Ac-RVRHA-CONH-benzyl | This paper | |
| Ac-RVRHA-CONH-methylbenzyl | This paper | |
| Ac-RVRHA-CONH-ethyloxyacetyl | This paper | |
| Ac-RVRHA-CONH-neopentyl | This paper | |
| Ac-RVRHA-CONH-pentyl | This paper | |
| Ac-RVRHA-CONH-phenylethyl | This paper | |
| Ac-RVRHA-CONH-(2,4-dimethyl)benzyl | This paper | |
| Ac-RVRHA-CONH-phenylbutyl | This paper | |
| Ac-RHA-CONH-phenylethyl | This paper | |
| Ac-RHA-CONH-phenylethyl | This paper | |
| Ac-A-CONH-phenylethyl | This paper | |
| Deposited Data | ||
| Crystal structure of GlpG bound to AcRVRHAcmk | This paper | PDB: 5MT8 |
| Crystal structure of GlpG bound to AcVRHAcmk | This paper | PDB: 5MT7 |
| Crystal structure of GlpG bound to Ac-RVRHA-CONH-phenylethyl | This paper | PDB: 5MT6 |
| Crystal structure of GlpG bound to Ac-RVRHA-CONH-(2,4-dimethyl)benzyl | This paper | PDB: 5MTF |
| Recombinant DNA | ||
| pMALp2E_MBP-LacYTM2-Trx | (Zoll et al., 2014) | pKS506 |
| pD881-SR | DNA2.0 Inc. | |
| pD881-SR_MBP-LacYTM2-Trx | This work | pPR61 |
| pET25b+M_GlpG | (Lemberg et al., 2005) | |
| pGP886_pxyl-AmyESP-MBP-FLAG-LacYTM2-Trx-HA | This work | pPR200 |
| Software and Algorithms | ||
| XDS | (Kabsch, 2010) | http://xds.mpimf-heidelberg.mpg.de/html_doc/downloading.html |
| AIMLESS | (Evans, 2011) | http://www.ccp4.ac.uk/html/aimless.html |
| COOT 0.8.6 | (Emsley and Cowtan, 2004) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| Jligand | (Lebedev et al., 2012) | http://www.ysbl.york.ac.uk/mxstat/JLigand/ |
| Refmac_5.8.0158 | (Murshudov et al., 2011) | |
| Ligplot+ | (Laskowski and Swindells, 2011) | http://www.ebi.ac.uk/thornton-srv/software/LigPlus/download.html |
| Phaser | (McCoy, 2007) | http://www.ccp4.ac.uk/html/phaser.html |
| Pymol 1.8.4.0 | Schrodinger, LLC | https://www.pymol.org/ |
| Turbomole 7 | (Ahlrichs et al., 1989) | http://www.turbomole.com/ |
| ImageJ 1.49 | (Schneider et al., 2012) | https://imagej.net/Welcome |
| Image Studio Lite Version 5.2 | LI-COR, Inc. | https://www.licor.com/bio/products/software/image_studio_lite/download.html |
| GraphPad Prism 7.02 | GraphPad Software, Inc. | https://www.graphpad.com/scientific-software/prism/ |
| GraFit 7 | Erithacus Software Ltd. | http://www.erithacus.com/grafit/ |
Contact for Reagents and Resource Sharing
Further information and requests for reagents may be directed to, and will be fulfilled by the corresponding author Kvido Strisovsky (kvido.strisovsky@uochb.cas.cz).
Experimental Model and Subject Details
Escherichia coli K12 strain NR698 (Ruiz et al., 2005), which has the MC4100 background with the imp4213 allele carried from BE100 (Eggert et al., 2001) (an in-frame deletion of amino acids 330-352 of LptD) is a gift of Dr. Tom Silhavy (Princeton University). A GlpG-free variant was created by deleting glpG using a tetracyclin marker (Pierrat et al., 2011).
To generate a rhomboid activity free Bacillus subtilis, the ydcA::neo mutant (BS2, this work) of the wild type B. subtilis 168 strain (Bacillus Genetic Stock Center, USA) was modified by deleting the entire the yqgP gene and replacing it with a tetracyclin resistance gene using homologous recombination, yielding strain BS4 (ydcA::neo, yqgP::tet). Both modifications were verified by genomic PCR of the disrupted locus and Sanger sequencing of the amplified region.
Method Details
Constructs and Cloning
To generate a model rhomboid substrate for in vivo activity assays in E. coli, the MBP-LacYTM2-Trx-Stag-Histag construct was PCR-amplified from pKS506 (Ticha et al., 2017) and cloned into the SapI linearized plasmid pD881-SR (DNA2.0 Inc., Newark, USA) using isothermal assembly (Gibson, 2011), yielding construct pPR61. For expression in B. subtilis, the substrate was modified by replacing the MBP signal peptide by the signal peptide from B. subtilis AmyE, and the AmyEsp-MBPmat-FLAG-LacYTM2-Trx-HA construct was cloned into the XbaI, SalI digested plasmid pGP886 (Gerwig et al., 2014) (gift of Dr. Libor Krasny, Prague, CR) using isothermal assembly (Gibson, 2011) to yield construct pPR200. This construct was linearized by ScaI and integrated into the xkdE locus of BS2 and BS4 using erythromycin-lincomycin selection yielding strains BS87 (BS2 xkdE::Pxyl-LacYTM2(erm)) and BS88 (BS4 xkdE::Pxyl-LacYTM2(erm)).
Protein Expression and Purification
The E. coli GlpG for crystallisation was expressed in E. coli C41(DE3) (Miroux and Walker, 1996) in PASM 5052 medium as described (Lee et al., 2014). Membrane isolation, purification by metal affinity chromatography, cleavage by chymotrypsin to produce GlpG transmembrane core domain and gel filtration chromatography were carried out as described previously (Vinothkumar et al., 2010, Zoll et al., 2014). The E. coli GlpG for inhibition studies was expressed in E. coli C41(DE3) (Miroux and Walker, 1996) in LB medium, and solubilised and purified in 0.05% (w/v) DDM as described (Ticha et al., 2017). Other rhomboid proteases were expressed and purified as reported previously (Wolf et al., 2015).
Chemical Synthesis
All reagents were acquired from commercial sources and used without purification. Protected amino acids and amino acid derivatives were purchased from Iris Biotech (Marktredwitz, Germany), Sigma-Aldrich (St. Louis, MO, U.S.A), Thermo Fischer Scientific (Waltham, Massachusets, U.S.A) and Fluorochem (Hadfield, Derbyshire, UK). Further details on chemical syntheses as well as compound characterisation data by mass spectrometry and NMR are available as Supplemental Information (Methods S1).
Protein Crystallography
Crystals of truncated wild type GlpG apoenzyme were obtained by mixing a solution of 2 - 3 M ammonium chloride or sodium chloride, 0.1 M Bis-Tris, pH 7.0 with protein (4-6 mg/mL) at ratio of 1:1 in hanging drops at 22°C (Vinothkumar et al., 2010, Wang and Ha, 2007). Inhibitors were diluted from 10 mM stock solutions in anhydrous DMSO into buffer resembling the mother liquor to yield final 1 mM inhibitor and 10% DMSO just before soaking. For the chloromethylketone inhibitors, the crystals were incubated with inhibitors at 0.3-0.5 mM concentrations for 24 h. The ketoamide inhibitors were incubated at final concentrations of 0.3-0.5 mM for 30-120 min. All crystals were cryo-protected by adding 25% (v/v) glycerol to the mother liquor and flash frozen in liquid nitrogen.
Data sets of the CMKs and 9 were collected at the I02 beam line at the Diamond Light Source (Harwell) and the data set of 10 was collected at BESSY (Berlin, Germany). Diffraction data were indexed, integrated and scaled with XDS (Kabsch, 2010) and AIMLESS (Evans, 2011). For the structures with inhibitor bound, the coordinates of GlpG (PDB 2XOV) with residues 245-249 (of Loop 5) omitted were used as an input model for Phaser (McCoy, 2007). Restrained refinement was carried out with Refmac (Murshudov et al., 2011) followed by manual model building in COOT (Emsley and Cowtan, 2004). In the final step, TLS was used using the enzyme and the inhibitor peptide as one group (Murshudov et al., 2011). The model, library and link files of the inhibitors were generated with Jligand (Lebedev et al., 2012). In the structures of Ac-(R)VRHA-cmk, H150 was modelled to hydrogen bond to the chloromethylketone oxygen. An additional density was observed close to M149 and H150 raising the possibility that the H150 residue could be also in an alternative conformation, but modeling the alternative conformation or both conformations of H150 was not conclusive in explaining the density. Other similar datasets of CMKs obtained by soaking show that this density might perhaps represent a bound ion, but due to ambiguity we have left the density unmodelled.
In order to find the best possible fit of the molecules of 9 and 10 to the experimental electron densities, quantum mechanical calculations were performed. The model systems comprised the whole inhibitors in their tetrahedral intermediate form with methoxy group representing the S201 side-chain. These models were made in several variants: i) cis/trans isomers of the ketoamide proximal/distal carbonyls, ii) cis/trans isomers of the distal carbonyl/NH, and iii) different rotameric forms of the His side chain in the P2 position of the inhibitors. All these variants were optimized in Turbomole ver. 7 program (Ahlrichs et al., 1989) using DFT-D3 method (Grimme, 2006) at B-LYP/DZVP level (Fanfrlik et al., 2016, Jensen, 2006) and COSMO implicit solvent model (Klamt and Schüürmann, 1993). Their intrinsic stabilities were assessed by comparing the final energies, and the conformer with the lowest energy was built into the electron density and chosen as a model for crystallographic refinement.
Noncovalent interactions between the ligands and protein were detected using Ligplot+ (Laskowski and Swindells, 2011) and hydrogen bonds were defined by canonical geometrical criteria (Laskowski and Swindells, 2011, McDonald and Thornton, 1994). Structural alignments and all structure figures were made with Pymol (Schrodinger, 2012). The coordinates of the structures presented in this manuscript have been deposited in the PDB under the following IDs: 5MT7 (Ac-VRHA-cmk), 5MT8 (Ac-RVRHA-cmk), 5MT6 (compound 9) and 5MTF (compound 10). Data collection and refinement statistics are listed in Table S1.
Rhomboid Activity and Inhibition Assays
The activity of GlpG in vitro was determined as reported (Ticha et al., 2017). Concentrations of stock solutions of peptide substrates and inhibitors were determined by quantitative amino acid analysis. The IC50 and reversibility measurements were performed in 20 mM HEPES, pH 7.4, 150 mM NaCl, 0.05%(w/v) DDM, 12%(v/v) DMSO, and other kinetic measurements in 50 mM potassium phosphate, pH 7.4, 150 mM NaCl, 0.05%(w/v) DDM, 10%(v/v) DMSO, 0.05%(w/v) PEG8000, and 20%(v/v) glycerol unless noted otherwise. The reaction mixture typically consisted of 10 μM fluorogenic peptide substrate and the measurements were performed without enzyme-inhibitor pre-incubation unless noted otherwise. Note that the fluorogenic substrates used in Figure 4 had nearly identical amino acid sequences but for the point of attachment of the fluorophore or the identity of the fluorophore and quencher (see Key Resources Table).
For measuring the inhibition of GlpG in vivo, the E. coli strain NR698 with genetically permeabilised outer membrane (Ruiz et al., 2005) and its glpG knock-out derivative KS69 (glpG::tet) were used as described (Pierrat et al., 2011) with the following modifications. The chimeric substrate encoding LacY transmembrane domain 2 inserted between maltose binding protein and thioredoxin (Strisovsky et al., 2009) was expressed under control of rhamnose promoter (construct pPR61). To evaluate the in vivo inhibition by ketoamides, the NR698 cells were inoculated to the density of OD600 = 0.05 and grown to OD600=0.6 at 37°C. The cells were then incubated with increasing concentrations of inhibitor for 15 min at room temperature, and expression of the chimeric substrate was induced by adding 1 mM L-rhamnose. Cells were grown for further 4 h at 25°C, after which steady-state level of substrate cleavage was evaluated by western blotting with near-infrared fluorescence detection as described (Ticha et al., 2017).
For measuring the inhibition of YqgP in vivo, the B. subtilis strains BS87 and its yqgP knock-out derivative BS88, generated in this work (see Constructs and Cloning section), were used as follows. The chimeric LacYTM2 substrate AmyEsp-MBP-FLAG-LacYTM2-Trx-HA (this work) was expressed under control of xylose promoter from the xdkE genomic locus. Fresh LB medium, supplemented with appropriate antibiotic, was inoculated with a few colonies of the B. subtilis strain grown overnight on selective LB agar plate and pre-culture was grown for 2 h at 37°C to OD600 = 1. Pre-culture was then diluted with fresh LB medium to the density of OD600 = 0.05. At this point, the expression of LacYTM2 was induced by adding 1% (w/v) D-(+)-xylose (Sigma), rhomboid inhibitors were added at a range of concentrations, and the cultures were further incubated for 2.5 h at 37°C (reaching OD600 ∼ 1). Steady-state conversion of the substrate was evaluated by western blotting with near-infrared fluorescence detection as described (Ticha et al., 2017), substracting the intensity of non-specific bands, closely co-migrating with the specific rhomboid-formed N-terminal cleavage product of the substrate.
Inhibitor Selectivity Profiling
For inhibitor selectivity profiling against rhomboid proteases (Wolf et al., 2015), 400 ng of a purified protein preparation of E. coli GlpG was diluted in 30 μL of reaction buffer (20 mM HEPES, pH 7.4, with 0.05% (w/v) DDM). For other rhomboids, amounts were taken that gave similar labeling intensity during profiling. Rhomboids were incubated for 30 min at room temperature with the indicated concentration of compound, 100 μM DCI as positive control, or an equal volume of DMSO as negative control. Next, TAMRA-FP serine hydrolase probe (Thermo Fisher #88318) was added to a final concentration of 1 ìM and incubated for 2 h at 37°C in the dark. The reaction was stopped by addition of 4× Laemmli buffer and the reaction mixture was resolved on 15% SDS-PAGE. Gels were scanned on a Typhoon Trio+ and analyzed using ImageJ. The intensity of each rhomboid protease band calculated by ImageJ was normalized against its corresponding DMSO-treated counterpart (100% activity) to indicate the residual activity left after inhibition. The remaining activity was used to calculate the percentage of inhibition depicted in the heatmap. Selectivity profiles against human serine hydrolases were determined by EnPlex as described previously (Bachovchin et al., 2014).
Quantification and Statistical Analysis
Enzyme kinetics and inhibition data were analysed in GraphPad Prism v7.02 using in-built algorithms. Means and standard deviations have been derived from the best fit of the data, or based on three independent measurements, as specified, unless noted otherwise. Quantitative western blots were evaluated using near infrared detection with the IRDye 800CW secondary antibody on a LiCor Odyssey CLx infrared scanner with normalisation to total protein using the Revert total protein stain (LiCor).
Data and Software Availability
All crystallographic coordinates of the protein structures presented in this manuscript have been deposited in and will be freely available from the Protein Data Bank (www.rcsb.org) under the following identifiers: 5MT7, 5MT8, 5MT6 and 5MTF.
Author Contributions
K.S. conceived and coordinated the study, designed and evaluated experiments, and wrote the paper with the input of the co-authors. P.M. and S.S. designed and S.S. performed all chemical syntheses, and A.T. designed, performed, and evaluated all kinetics and inhibition measurements with the help of K.Š. and J.Š. in the initial stages. K.R.V., D.C.M., and P.P. performed all crystallographic experiments and evaluated the data with the input of M.L. M.T.N.N. and S.H.L.V. performed selectivity profiling against rhomboid proteases, J.B. performed all experiments on B. subtilis, and D.C.J. and D.A.B. performed and evaluated the EnPlex experiments.
Acknowledgments
We thank Mirka Blechová and Lenka Monincová for peptide synthesis and HPLC/MS analyses, Radko Souček for amino acid analysis, Jiří Brynda for help with crystallographic data analysis, Pavel Srb and Marek Ingr for help and advice on data fitting and kinetics, Petra Rampírová for DNA cloning and laboratory assistance, Libor Krásný and Tom Silhavy for reagents and advice, and Colin Adrain and Cyril Bařinka for critical reading of the manuscript. K.S. was a recipient of a Purkyne Fellowship of the Academy of Sciences of the Czech Republic and acknowledges support also from EMBO (installation grant no. 2329), Ministry of Education, Youth and Sports of the Czech Republic (project nos. LK11206 and LO1302), Marie Curie Career Integration Grant (project no. 304154), Gilead Sciences & IOCB Research Centre, and the National Subvention for Development of Research Organisations (RVO: 61388963) to the Institute of Organic Chemistry and Biochemistry. P.P. acknowledges support from the Ministry of Education of the Czech Republic (program “NPU I”), project no. LO1304, and J.S. and J.B. acknowledge support from the Grant Agency of Charles University (GA UK) in Prague (PhD grant project nos. 232313 and 170214). K.R.V. was supported by an MRC grant (MC_U105184322) as part of R. Henderson's group. D.A.B. was supported by Josie Robertson Foundation and the MSKCC (core grant P30 CA008748), and D.C.J. by the NIH (T32 GM115327-Tan). M.L. was supported by the Czech Science Foundation (grant number P208/12/G016). M.T.N.N. and S.H.L.V. are supported by the Deutsche Forschungsgemeinschaft and the Ministerium für Innovation, Wissenschaft und Forschung des Landes Nordrhein-Westfalen.
Published: October 26, 2017
Footnotes
Supplemental Information includes one table and supplemental text and can be found with this article online at https://doi.org/10.1016/j.chembiol.2017.09.007.
Supporting Citations
The following references appear in the Supplemental Information: Bastiaans et al., 1997, Cao et al., 2010, Coste et al., 1994, D'Andrea and Scola, 2008, Dondoni and Perrone, 1993, Semple et al., 2000, Souček and Urban, 1995, Tulla-Puche et al., 2008, Venkatraman et al., 2006, Yin et al., 2007.
Supplemental Information
References
- Ahlrichs R., Bär M., Häser M., Horn H., Kölmel C. Electronic structure calculations on workstation computers: the program system turbomole. Chem. Phys. Lett. 1989;162:165–169. [Google Scholar]
- Bachovchin D.A., Koblan L.W., Wu W., Liu Y., Li Y., Zhao P., Woznica I., Shu Y., Lai J.H., Poplawski S.E. A high-throughput, multiplexed assay for superfamily-wide profiling of enzyme activity. Nat. Chem. Biol. 2014;10:656–663. doi: 10.1038/nchembio.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R.P., Wijetilaka R., Urban S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PLoS Pathog. 2006;2:e113. doi: 10.1371/journal.ppat.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaans H.M.M., vanderBaan J.L., Ottenheijm H.C.J. Flexible and convergent total synthesis of cyclotheonamide B. J. Org. Chem. 1997;62:3880–3889. [Google Scholar]
- Cao H., Liu H., Domling A. Efficient multicomponent reaction synthesis of the schistosomiasis drug praziquantel. Chemistry. 2010;16:12296–12298. doi: 10.1002/chem.201002046. [DOI] [PubMed] [Google Scholar]
- Chan E.Y., McQuibban G.A. The mitochondrial rhomboid protease: its rise from obscurity to the pinnacle of disease-relevant genes. Biochim. Biophys. Acta. 2013;1828:2916–2925. doi: 10.1016/j.bbamem.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Dunn D., Tao M., Wells G., Gu Z.Q., Bihovsky R., Ator M.A., Siman R., Mallamo J.P. P2-achiral, P'-extended alpha-ketoamide inhibitors of calpain I. Bioorg. Med. Chem. Lett. 1999;9:2371–2374. doi: 10.1016/s0960-894x(99)00392-3. [DOI] [PubMed] [Google Scholar]
- Cho S., Dickey S.W., Urban S. Crystal structures and inhibition kinetics reveal a two-stage catalytic mechanism with drug design implications for rhomboid proteolysis. Mol. Cell. 2016;61:329–340. doi: 10.1016/j.molcel.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.T. A pivotal role for PINK1 and autophagy in mitochondrial quality control: implications for Parkinson disease. Hum. Mol. Genet. 2010;19:R28–R37. doi: 10.1093/hmg/ddq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland R.A. John Wiley; 2013. Reversible modes of inhibitor interactions with enzymes. In Evaluation of Enzyme Inhibitors in Drug Discovery; pp. 57–121. [Google Scholar]
- Copeland R.A. John Wiley; 2013. Slow binding inhibitors. In Evaluation of Enzyme Inhibitors in Drug Discovery; pp. 203–244. [Google Scholar]
- Coste J., Frerot E., Jouin P. Coupling N-methylated amino-acids using pybrop and pyclop halogenophosphonium salts - mechanism and fields of application. J. Org. Chem. 1994;59:2437–2446. [Google Scholar]
- D'Andrea, S., and Scola, P.M.. (2008). Inhibitors of hepatitis C virus. Bristol-Myers Squibb Company. US patent US2008107623 (A1), filed October 25, 2007, and published May 8, 2008.
- Dickey S.W., Baker R.P., Cho S., Urban S. Proteolysis inside the membrane is a rate-governed reaction not driven by substrate affinity. Cell. 2013;155:1270–1281. doi: 10.1016/j.cell.2013.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondoni A., Perrone D. 2-Thiazolyl alpha-amino ketones - a new class of reactive intermediates for the stereocontrolled synthesis of unusual amino-acids. Synthesis (Stuttg) 1993:1162–1176. [Google Scholar]
- Drag M., Salvesen G.S. Emerging principles in protease-based drug discovery. Nat. Rev. Drug Discov. 2010;9:690–701. doi: 10.1038/nrd3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert U.S., Ruiz N., Falcone B.V., Branstrom A.A., Goldman R.C., Silhavy T.J., Kahne D. Genetic basis for activity differences between vancomycin and glycolipid derivatives of vancomycin. Science. 2001;294:361–364. doi: 10.1126/science.1063611. [DOI] [PubMed] [Google Scholar]
- Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Evans P.R. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011;67:282–292. doi: 10.1107/S090744491003982X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanfrlik J., Holub J., Ruzickova Z., Rezac J., Lane P.D., Wann D.A., Hnyk D., Ruzicka A., Hobza P. Competition between halogen, hydrogen and dihydrogen bonding in brominated carboranes. ChemPhysChem. 2016;17:3373–3376. doi: 10.1002/cphc.201600848. [DOI] [PubMed] [Google Scholar]
- Fleig L., Bergbold N., Sahasrabudhe P., Geiger B., Kaltak L., Lemberg M.K. Ubiquitin-dependent intramembrane rhomboid protease promotes ERAD of membrane proteins. Mol. Cell. 2012;47:558–569. doi: 10.1016/j.molcel.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Gerwig J., Kiley T.B., Gunka K., Stanley-Wall N., Stulke J. The protein tyrosine kinases EpsB and PtkA differentially affect biofilm formation in Bacillus subtilis. Microbiology. 2014;160:682–691. doi: 10.1099/mic.0.074971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D.G. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 2011;498:349–361. doi: 10.1016/B978-0-12-385120-8.00015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimme S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006;27:1787–1799. doi: 10.1002/jcc.20495. [DOI] [PubMed] [Google Scholar]
- Harper J.W., Powers J.C. Reaction of serine proteases with substituted 3-alkoxy-4-chloroisocoumarins and 3-alkoxy-7-amino-4-chloroisocoumarins: new reactive mechanism-based inhibitors. Biochemistry. 1985;24:7200–7213. doi: 10.1021/bi00346a028. [DOI] [PubMed] [Google Scholar]
- Harper J.W., Hemmi K., Powers J.C. Reaction of serine proteases with substituted isocoumarins: discovery of 3,4-dichloroisocoumarin, a new general mechanism based serine protease inhibitor. Biochemistry. 1985;24:1831–1841. doi: 10.1021/bi00329a005. [DOI] [PubMed] [Google Scholar]
- Hedstrom L. Serine protease mechanism and specificity. Chem. Rev. 2002;102:4501–4524. doi: 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- Jensen F. Wiley; 2006. Introduction to Computational Chemistry. [Google Scholar]
- Kabsch W. Xds. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamt A., Schüürmann G. COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993;2:799–805. [Google Scholar]
- Krissinel E., Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., Swindells M.B. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Lebedev A.A., Young P., Isupov M.N., Moroz O.V., Vagin A.A., Murshudov G.N. JLigand: a graphical tool for the CCP4 template-restraint library. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012;68:431–440. doi: 10.1107/S090744491200251X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Kang H.J., Hjelm A., Qureshi A.A., Nji E., Choudhury H., Beis K., de Gier J.W., Drew D. MemStar: a one-shot Escherichia coli-based approach for high-level bacterial membrane protein production. FEBS Lett. 2014;588:3761–3769. doi: 10.1016/j.febslet.2014.08.025. [DOI] [PubMed] [Google Scholar]
- Lemberg M.K., Menendez J., Misik A., Garcia M., Koth C.M., Freeman M. Mechanism of intramembrane proteolysis investigated with purified rhomboid proteases. EMBO J. 2005;24:464–472. doi: 10.1038/sj.emboj.7600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.W., Meireles P., Prudencio M., Engelmann S., Annoura T., Sajid M., Chevalley-Maurel S., Ramesar J., Nahar C., Avramut C.M. Loss-of-function analyses defines vital and redundant functions of the Plasmodium rhomboid protease family. Mol. Microbiol. 2013;88:318–338. doi: 10.1111/mmi.12187. [DOI] [PubMed] [Google Scholar]
- Liu Y., Stoll V.S., Richardson P.L., Saldivar A., Klaus J.L., Molla A., Kohlbrenner W., Kati W.M. Hepatitis C NS3 protease inhibition by peptidyl-alpha-ketoamide inhibitors: kinetic mechanism and structure. Arch. Biochem. Biophys. 2004;421:207–216. doi: 10.1016/j.abb.2003.11.013. [DOI] [PubMed] [Google Scholar]
- McCoy A.J. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007;63:32–41. doi: 10.1107/S0907444906045975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald I.K., Thornton J.M. Satisfying hydrogen bonding potential in proteins. J. Mol. Biol. 1994;238:777–793. doi: 10.1006/jmbi.1994.1334. [DOI] [PubMed] [Google Scholar]
- Meissner C., Lorenz H., Hehn B., Lemberg M.K. Intramembrane protease PARL defines a negative regulator of PINK1- and PARK2/Parkin-dependent mitophagy. Autophagy. 2015;11:1484–1498. doi: 10.1080/15548627.2015.1063763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroux B., Walker J.E. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 1996;260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- Mitchell E.M., Artymiuk P.J., Rice D.W., Willett P. Use of techniques derived from graph-theory to compare secondary structure motifs in proteins. J. Mol. Biol. 1990;212:151–166. doi: 10.1016/0022-2836(90)90312-A. [DOI] [PubMed] [Google Scholar]
- Morrison J.F. The slow-binding and slow, tight-binding inhibition of enzyme-catalysed reactions. Trends Biochem. Sci. 1982;7:102–105. [Google Scholar]
- Murshudov G.N., Skubak P., Lebedev A.A., Pannu N.S., Steiner R.A., Nicholls R.A., Winn M.D., Long F., Vagin A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M.T., Van Kersavond T., Verhelst S.H. Chemical tools for the study of intramembrane proteases. ACS Chem. Biol. 2015;10:2423–2434. doi: 10.1021/acschembio.5b00693. [DOI] [PubMed] [Google Scholar]
- Njoroge F.G., Chen K.X., Shih N.Y., Piwinski J.J. Challenges in modern drug discovery: a case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection. Acc. Chem. Res. 2008;41:50–59. doi: 10.1021/ar700109k. [DOI] [PubMed] [Google Scholar]
- O'Donnell R.A., Hackett F., Howell S.A., Treeck M., Struck N., Krnajski Z., Withers-Martinez C., Gilberger T.W., Blackman M.J. Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite. J. Cell Biol. 2006;174:1023–1033. doi: 10.1083/jcb.200604136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue A.J., Eroy-Reveles A.A., Knudsen G.M., Ingram J., Zhou M., Statnekov J.B., Greninger A.L., Hostetter D.R., Qu G., Maltby D.A. Global identification of peptidase specificity by multiplex substrate profiling. Nat. Methods. 2012;9:1095–1100. doi: 10.1038/nmeth.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrat O.A., Strisovsky K., Christova Y., Large J., Ansell K., Bouloc N., Smiljanic E., Freeman M. Monocyclic beta-lactams are selective, mechanism-based inhibitors of rhomboid intramembrane proteases. ACS Chem. Biol. 2011;6:325–335. doi: 10.1021/cb100314y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J.C., Kam C.M., Narasimhan L., Oleksyszyn J., Hernandez M.A., Ueda T. Mechanism-based isocoumarin inhibitors for serine proteases: use of active site structure and substrate specificity in inhibitor design. J. Cell. Biochem. 1989;39:33–46. doi: 10.1002/jcb.240390105. [DOI] [PubMed] [Google Scholar]
- Powers J.C., Asgian J.L., Ekici O.D., James K.E. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem. Rev. 2002;102:4639–4750. doi: 10.1021/cr010182v. [DOI] [PubMed] [Google Scholar]
- Purich D.L. Elsevier; 2010. Kinetic behavior of enzyme inhibitors. In Enzyme Kinetics: Catalysis & Control; pp. 485–574. [Google Scholar]
- Ruiz N., Falcone B., Kahne D., Silhavy T.J. Chemical conditionality. Cell. 2005;121:307–317. doi: 10.1016/j.cell.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrodinger . Schrödinger LLC; 2012. The PyMOL Molecular Graphics System, Version 1.5.0.4. [Google Scholar]
- Semple J.E., Owens T.D., Nguyen K., Levy O.E. New synthetic technology for efficient construction of alpha-hydroxy-beta-amino amides via the Passerini reaction. Org. Lett. 2000;2:2769–2772. doi: 10.1021/ol0061485. [DOI] [PubMed] [Google Scholar]
- Serim S., Haedke U., Verhelst S.H. Activity-based probes for the study of proteases: recent advances and developments. ChemMedChem. 2012;7:1146–1159. doi: 10.1002/cmdc.201200057. [DOI] [PubMed] [Google Scholar]
- Singh J., Petter R.C., Baillie T.A., Whitty A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- Song W., Liu W., Zhao H., Li S., Guan X., Ying J., Zhang Y., Miao F., Zhang M., Ren X. Rhomboid domain containing 1 promotes colorectal cancer growth through activation of the EGFR signalling pathway. Nat. Commun. 2015;6:8022. doi: 10.1038/ncomms9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souček M., Urban J. An efficient method for preparation of optically active N-protected α-amino aldehydes from N-protected α-amino alcohols. Collect. Czechoslov. Chem. Commun. 1995;60:693–696. [Google Scholar]
- Stevenson L.G., Strisovsky K., Clemmer K.M., Bhatt S., Freeman M., Rather P.N. Rhomboid protease AarA mediates quorum-sensing in Providencia stuartii by activating TatA of the twin-arginine translocase. Proc. Natl. Acad. Sci. USA. 2007;104:1003–1008. doi: 10.1073/pnas.0608140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strisovsky K. Structural and mechanistic principles of intramembrane proteolysis - lessons from rhomboids. FEBS J. 2013;280:1579–1603. doi: 10.1111/febs.12199. [DOI] [PubMed] [Google Scholar]
- Strisovsky K. Rhomboid protease inhibitors: emerging tools and future therapeutics. Semin. Cell Dev. Biol. 2016;60:52–62. doi: 10.1016/j.semcdb.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Strisovsky K. Why cells need intramembrane proteases - a mechanistic perspective. FEBS J. 2016;283:1837–1845. doi: 10.1111/febs.13638. [DOI] [PubMed] [Google Scholar]
- Strisovsky K., Sharpe H.J., Freeman M. Sequence-specific intramembrane proteolysis: identification of a recognition motif in rhomboid substrates. Mol. Cell. 2009;36:1048–1059. doi: 10.1016/j.molcel.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticha A., Stanchev S., Skerle J., Began J., Ingr M., Svehlova K., Polovinkin L., Ruzicka M., Bednarova L., Hadravova R. Sensitive versatile fluorogenic transmembrane peptide substrates for rhomboid intramembrane proteases. J. Biol. Chem. 2017;292:2703–2713. doi: 10.1074/jbc.M116.762849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulla-Puche J., Torres A., Calvo P., Royo M., Albericio F. N,N,N',N'-Tetramethylchloroformamidinium hexafluorophosphate (TCFH), a powerful coupling reagent for bioconjugation. Bioconj. Chem. 2008;19:1968–1971. doi: 10.1021/bc8002327. [DOI] [PubMed] [Google Scholar]
- Venkatraman S., Bogen S.L., Arasappan A., Bennett F., Chen K., Jao E., Liu Y.T., Lovey R., Hendrata S., Huang Y. Discovery of (1R,5S)-N-[3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]- 3-[2(S)-[[[(1,1-dimethylethyl)amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]- 6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2(S)-carboxamide (SCH 503034), a selective, potent, orally bioavailable hepatitis C virus NS3 protease inhibitor: a potential therapeutic agent for the treatment of hepatitis C infection. J. Med. Chem. 2006;49:6074–6086. doi: 10.1021/jm060325b. [DOI] [PubMed] [Google Scholar]
- Vinothkumar K.R., Strisovsky K., Andreeva A., Christova Y., Verhelst S., Freeman M. The structural basis for catalysis and substrate specificity of a rhomboid protease. EMBO J. 2010;29:3797–3809. doi: 10.1038/emboj.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinothkumar K.R., Pierrat O.A., Large J.M., Freeman M. Structure of rhomboid protease in complex with beta-lactam inhibitors defines the S2' cavity. Structure. 2013;21:1051–1058. doi: 10.1016/j.str.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosyka O., Vinothkumar K.R., Wolf E.V., Brouwer A.J., Liskamp R.M., Verhelst S.H. Activity-based probes for rhomboid proteases discovered in a mass spectrometry-based assay. Proc. Natl. Acad. Sci. USA. 2013;110:2472–2477. doi: 10.1073/pnas.1215076110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B., Lynas J.F. Strategies for the inhibition of serine proteases. Cell. Mol. Life Sci. 2001;58:596–624. doi: 10.1007/PL00000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Ha Y. Open-cap conformation of intramembrane protease GlpG. Proc. Natl. Acad. Sci. USA. 2007;104:2098–2102. doi: 10.1073/pnas.0611080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang Y., Ha Y. Crystal structure of a rhomboid family intramembrane protease. Nature. 2006;444:179–180. doi: 10.1038/nature05255. [DOI] [PubMed] [Google Scholar]
- Wolf E.V., Zeissler A., Vosyka O., Zeiler E., Sieber S., Verhelst S.H. A new class of rhomboid protease inhibitors discovered by activity-based fluorescence polarization. PLoS One. 2013;8:e72307. doi: 10.1371/journal.pone.0072307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E.V., Zeissler A., Verhelst S.H. Inhibitor fingerprinting of rhomboid proteases by activity-based protein profiling reveals inhibitor selectivity and rhomboid autoprocessing. ACS Chem. Biol. 2015;10:2325–2333. doi: 10.1021/acschembio.5b00514. [DOI] [PubMed] [Google Scholar]
- Xue Y., Chowdhury S., Liu X., Akiyama Y., Ellman J., Ha Y. Conformational change in rhomboid protease GlpG induced by inhibitor binding to its S' subsites. Biochemistry. 2012;51:3723–3731. doi: 10.1021/bi300368b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Gallis C.E., Chisholm J.D. Tandem oxidation/halogenation of aryl allylic alcohols under Moffatt-Swern conditions. J. Org. Chem. 2007;72:7054–7057. doi: 10.1021/jo0711992. [DOI] [PubMed] [Google Scholar]
- Zoll S., Stanchev S., Began J., Skerle J., Lepsik M., Peclinovska L., Majer P., Strisovsky K. Substrate binding and specificity of rhomboid intramembrane protease revealed by substrate-peptide complex structures. EMBO J. 2014;33:2408–2421. doi: 10.15252/embj.201489367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.