Figure 3.
Modification of the Prime-Side Substituent at the Amide Group of Peptidyl Ketoamides Enhances Their Potency by Orders of Magnitude
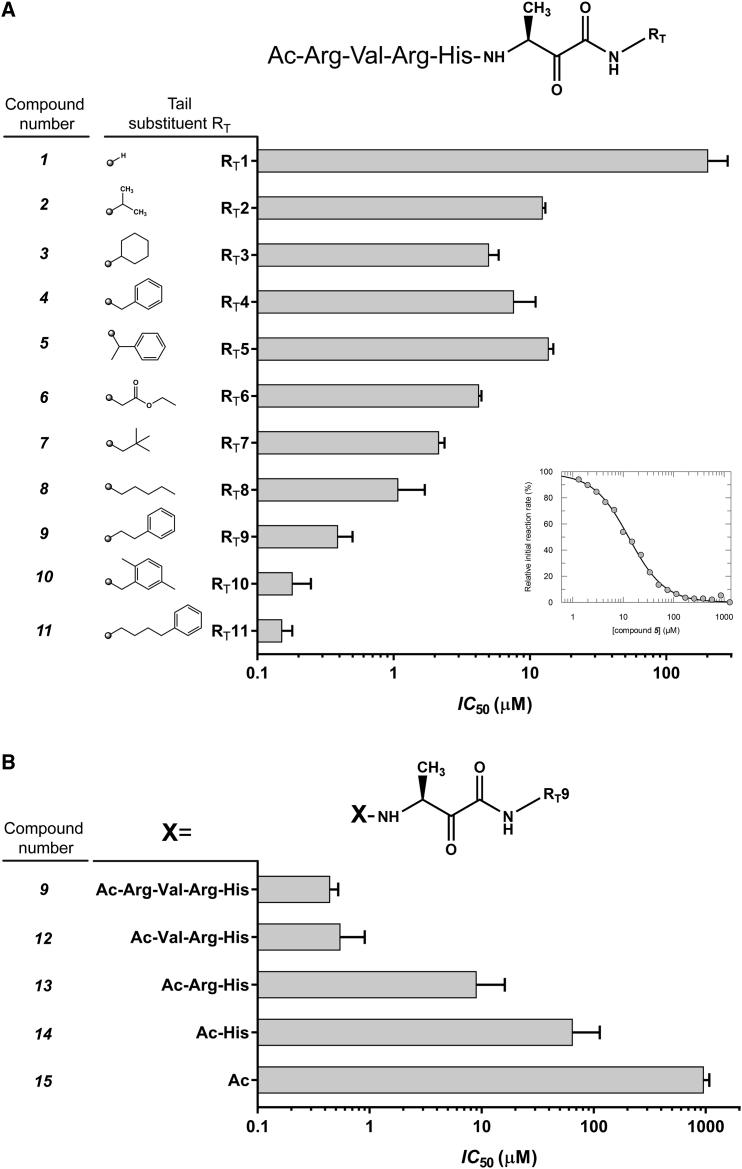
(A) A screen of the effect of the tail substituent RT on the inhibitory properties of ketoamide inhibitors of GlpG based on the parent compound Ac-RVRHA-CONH2. The apparent IC50 values of all compounds were measured in 0.05% (w/v) DDM and 10 μM KSp35 (Ticha et al., 2017) with 1 hr preincubation. The IC50 values of the most effective compounds 9, 10, and 11 are three orders of magnitude lower than that of the parent compound 1. The reported values are best-fit means with SD representative of 2–3 measurements. The inset shows a typical inhibition curve.
(B) The significance of the peptidyl part in compound 9. The peptidyl part of 9 was progressively truncated from the N terminus, and the apparent IC50 values of all compounds were measured in 0.05% (w/v) DDM and 10 μM KSp35 (Ticha et al., 2017) with 1 hr preincubation. The reported values are best-fit means with SD representative of 2–3 measurements.