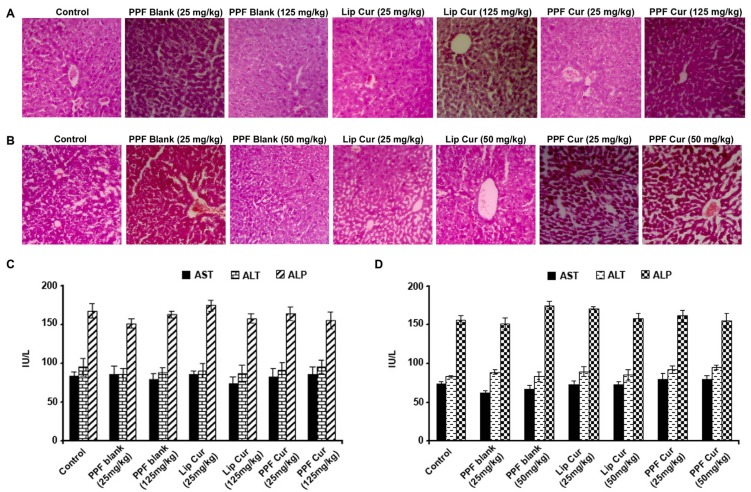
Figure 3. PPF curcumin is pharmacologically safe as assessed by acute and chronic toxicity studies in Swiss albino mice.
(A) Histopathological analysis of liver tissues of mice administered with 25 mg/kg or 125 mg/kg liposomal curcumin, PPF-curcumin or the void carrier, during acute toxicity study (7 days). (B) Histopathological analysis of the liver tissues of mice subjected to chronic toxicity study for 2 months using 25 mg/kg or 50 mg/kg liposomal curcumin, PPF-curcumin or void carrier. (C) Liver function parameters of mice subjected to acute toxicity study using 25 mg/kg or 125 mg/kg liposomal curcumin, PPF-curcumin or the void carrier. (D) Liver function parameters of mice subjected to chronic toxicity study using 25 mg/kg or 50 mg/kg liposomal curcumin, PPF-curcumin or the void carrier.