Abstract
Background
Socioeconomic inequalities in non-communicable diseases are known to exist; however there is a paucity of research describing the secular trends in these inequalities. To this end, the current study aims to explore the recent time trends in social patterning of selected non-communicable diseases among Korean adults between 2007 and 2015.
Methods
Using data from the Korea National Health and Nutrition Examination Survey (KNHANES), temporal trends in socioeconomic inequalities in diabetes, arthritis, asthma and depressive symptoms were assessed across three time points. Respondents were adults aged 20 years or over (N = 47,091, 20,180 men and 26,911 women). Socioeconomic circumstance was assessed based on highest level of educational attainment. We estimated prevalence ratios with 95% confidence intervals using Poisson regression with robust variance estimation (adjusted for age, smoking status, alcohol consumption, obesity, and physical activity) separately for men and women. The magnitude of the inequalities was computed using the relative index of inequality (RII).
Results
In men, diabetes was not associated with educational attainment, while there was evidence of a negative association in women across surveys. Similar inverse associations were found with arthritis and depressive symptoms, but these associations were less clear for asthma. RII showed a non-significant increasing trend in educational disparities in depressive symptoms. Meanwhile, relative inequalities in diabetes, arthritis and asthma have narrowed. These trends were, in general, more pronounced in women.
Conclusions
The findings of this study indicate higher burden of selected NCDs among the lower educational groups, particularly among women. In addition, our results indicated some improvements in inequalities in diabetes, arthritis and asthma in recent years. These findings have important implications for understanding the causes of social patterning of NCDs and for the targeting of effective interventions.
Introduction
Cardiovascular disease, diabetes, chronic respiratory diseases, and other non-communicable diseases (NCDs) are major public health concern, being the leading causes of mortality worldwide. Of the 56.4 million deaths that occurred globally in 2015, an estimated 39.5 million–approximately 70%–were due to NCDs and, by 2030, total deaths attributable to NCDs are projected to increase substantially to 52 million [1]. NCDs have long been considered as problem of high income countries; however, the percentage of total disease burden attributable to non-communicable is growing rapidly in the Asia-Pacific region [2]. In the case of Korea, NCDs are the leading contributors to mortality and disability-adjusted life years, accounting for almost 80% of all deaths and 85% of total disability-adjusted life-years (DALYs) lost in 2012 [3, 4].
Over the past few decades, Korea has experienced unprecedented economic development which has resulted in substantial unequal distribution of income opportunities, health care resources and deepening social polarization [5, 6]. In this context, tackling inequalities in health has featured prominently as a public health priority in recent years [7]. Much research has been devoted to elucidating the relationship between socioeconomic status and non-communicable diseases [8–12]. To date however, few studies have empirically examined the time trends in socioeconomic patterning of NCDs. In particular, existing studies have mostly focused on outcomes such as mortality [13–15], self-rated health [16, 17] and fatal diseases like cancer [18, 19] and cardiovascular diseases including stroke [20]. Yet the extent of and the trends in socioeconomic inequality with respect to nonfatal diseases, such as arthritis and asthma, despite being one of the leading contributors to Quality-Adjusted Life-years (QALYs) lost in Korea, have received relatively little attention [21]. Given the increasing burden of chronic diseases, monitoring the dynamics of the relationship between socioeconomic status (SES) and non-communicable diseases over time is imperative for development of targeted interventions aimed at tackling non-communicable diseases and improving ones’ quality of life. Thus, this study aims to investigate the recent trends in educational inequalities in selected non-fatal NCDs among Korean adults. Further, the gender differences in these inequalities were explored.
Materials and methods
Data
This study was based on data from the Korea National Health and Nutrition Examination Survey (KNHANES). KNHANES is an ongoing cross-sectional survey of non-institutionalized adults resident in Korea, commissioned by the Korea Centers for Disease Control and Prevention (KCDC). The first three KNHANES surveys (1998, 2001, 2005) were initially implemented as a tri-annual survey. Beginning with the fourth KNHANES in 2007, it was converted to an annual survey to provide timely national statistics on health [22]. The details of the sampling procedure and KNHANES survey response rates have been provided elsewhere [23]. In brief, the complete survey, which consists of three components (health interview, health examination survey, and a nutrition survey), covers a comprehensive range of topics including demographic and socio-economic characteristics, diet and nutrition, healthcare utilization, and general health status. To obtain a representative sample of the Korean population, this survey used a multi-stage stratified probability sampling procedure based on the geographic area and administrative districts drawn from household registries. All individuals voluntarily agreed to participate and provided written informed consent prior to enrollment.
For consistency in the methodology of data collection among the study periods, only data from years 2007 to 2015 were used in our analyses. The overall data was divided into three 3-year periods (2007–2009; 2010–2012; 2013–2015) to obtain robust estimates for each time period. From an original sample of 73,353 individuals, we excluded from our analysis individuals younger than 20 years (n = 17,969). An additional 8,293 cases were excluded due to missing data on outcomes and educational attainment. After these exclusions, total sample of 47,091 subjects (20,180 men; 26,911 women) were available for analysis. Compared with the analytic sample, those excluded from the sample because of missing data tended to be older (mean 53.5 years vs. 49.9 vs.; p<0.001), more likely to be male (45.1% vs. 42.9%; p<0.001), and have lower levels of formal schooling (Elementary education 43.8% vs. 23.2%;p<0.001).
Ethical statement
Data from the KNHANES survey are made publicly available through the KNHANES website (http://knhanes.cdc.go.kr). Thus, ethical approval was not required for this study.
Variables
Definition of non-communicable diseases (NCD)
The main outcomes of interest in the study were diabetes mellitus, asthma, arthritis, and depressive symptoms. Presence of asthma and arthritis were ascertained through self-report of physician diagnosis of each disease during the health interview. Depressive symptoms were assessed through responses to a single-item question: “During the past 12 months, have you ever felt sad or hopeless and been unable to complete daily activities for two weeks or more?” (Yes or no). Diabetes was defined according to the following criteria: fasting plasma glucose levels ≥ 126 mg/dL, current use of anti-diabetic medications, or a previous diagnosis of diabetes by a physician.
Measure of socioeconomic circumstance
We used educational attainment as a measure of socioeconomic circumstances. In the KNHANES, the participants were asked the level at which their education was completed; for this analysis, participants were placed into four educational groups: (1) completion of elementary school, (2) middle school, (3) high school and (4) university level or above.
Covariates
Detailed information on the socio-demographic characteristics of participants was collected by trained staff through standardised questionnaires. Age was categorized as (1) 20–29 years, (2) 30–39 years, (3) 40–49 years, (4) 50–59 years, and (5) 60+ years. Among health-related behaviours, smoking, alcohol intake, physical activity, and obesity are typically more prevalent among those in the lower socioeconomic stratum and are associated with health-related outcomes [24–26]. We therefore considered them as covariates in our analysis. Participants were classified into three groups according to their smoking status: (1) Never smoker (Those who had never smoked or had smoked less than 100 cigarettes in their lifetime), (2) Former smoker (Those who reported smoking at least 100 cigarettes in their lifetime and who did not smoke at all at the time of the survey), (3) Current smokers (Subjects who reported smoking at least 100 cigarettes in their lifetime and who smoked either every day or on some days at the time of the survey). Participants were asked to indicate if they had consumed at least one glass of alcohol in their lifetime, recorded using a dichotomous (yes or no) scale. Physical activity was defined as engaging in moderate physical activity at least 5 days per week for more than 30 minutes each day or strenuous physical activity performed for at least 20 min at a time at least three times per week. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared, and categorized in three groups using the criteria for Asians developed by the Western Pacific Region Office of the World Health Organization [27]. Specifically, individuals were classified as less than 18.5 kg/m2 (underweight), between 18.5 and 24.9 kg/m2 (normal weight), and more than 25 kg/m2 (obese).
Statistical analysis
Previous studies have demonstrated significant gender differences in the association between socioeconomic status and chronic diseases [28–30]. Hence all analyses were performed separately for men and women. Distributions of study variables are summarized as weighted percentages for categorical variables and weighted means (standard errors) for continuous variables. Age-standardized estimates of NCD prevalence was initially computed for each time period, overall and according to socioeconomic groups, using the direct standardisation method. The Korean 2010 census population was used as the standard population. Subsequently, Poisson regression with robust variance estimation (a generalized linear model with a logarithmic link function and Poisson distribution) were used to estimate prevalence ratios (PRs) and 95% confidence intervals for each NCD across educational groups [31, 32]. By assuming that the observations are independent and log transformed outcomes are linearly related to the independent variable, exponentiation of the model coefficients yields the PR. PRs are preferred to odds ratios because they are more easily interpretable when evaluating exposure effect [33] and since it is known that odds ratios overestimate the PRs, particularly when the outcome is not rare [34]. Model 1 was adjusted for age only; in model 2, we additionally adjusted for potentially mediating behavioural risk factors (self-reported smoking status, alcohol consumption, obesity, and physical activity). The highest educational level group was used as the reference and P values of less than 0.05 were considered statistically significant. For the behavioural risk factors, there were small amounts of missing values, usually less than 1%. These observations were treated as a separate missing category and included in the models to enable inclusion of all participants.
In addition, the extent of educational inequalities in NCDs was measured using the relative index of inequality (RII) [35]. The RII is a continuous measure of educational inequalities that accounts simultaneously for the changing size and relative position of educational groups across time. Educational attainment is hierarchically ranked from highest to lowest and a ridit-score is derived for each stratum of educational level by calculating the mean proportion of the population with a higher SES. In our case, for instance, if the university level and high school education groups comprise 20% and 10% of the population, using midpoints for the proportion of the population in each stratum, the ridit-score for those with university level education would be 0.1 (0.2/2) and 0.25 (0.2 +(0.1/2)) for those attaining high school education only. Each of the outcomes was then regressed on these ridit-scores, with the resulting regression coefficient representing the RII. RII values larger than 1 imply that the prevalence of NCDs is higher in the lower socioeconomic group. Additionally, in order to assess the changes in RII across surveys, an interaction term of ridit-score*survey was included in each model. Also, gender differences in inequality for each survey were assessed by inserting two-way interaction terms (Ridit-score*sex), using men as the reference group. All statistical analyses were performed using survey estimation command (svy) of STATA, version 13 (StataCorp, College Station, TX) to incorporate sample weights and account for clusters and strata of the complex sample design.
Results
Sample characteristics
Table 1 gives the socio-demographic characteristics of participants in each KNHANES survey. The total sample comprised of 47,091 respondents, including 20,180 men and 26,911 women. Over the study period, there was a steady rise in the mean age for both sexes; mean age increased from 43.6 years (SD = 0.27) in 2007–2009 to 45.1 years (SD = 0.26) in 2013–2015, and from 45.4 years (SD = 0.28) in 2007–2009 to 46.7 years (SD = 0.27) in 2013–2015 for men and women, respectively. The proportion of women was greater than that of men in all surveys, with females constituting approximately 57% of the total sample. The majority of the participants were married, and physically inactive. Approximately one-third (between 36.3–38.4% males; 26.6–28.4% females) of the sample was classified as obese. The proportion of current smokers in the surveyed population ranged between 41.0% and 47.3% in men, and 5.7% and 7.0% in women, whereas the percentage of current drinkers among males and females ranged around 95% of men and 82–85% of women. In both men and women, the percentage of participants with elementary school education declined between 2007–2009 and 2013–2015, whilst those reporting a university education increased over the study period. Regarding the four selected NCDs, the weighted proportion of adults reporting diabetes ranged from 8.5% to 10.6% in men, and in women from 6.9% to 7.8%. For depressive symptoms, the values ranged between 5.3% to 9.3% in men and 10.2% to 18.6% in women. Among males, the crude prevalence of physician-diagnosed arthritis ranged from 3.0% to 4.2% across surveys, and in females from 12.9% to 13.2%, meanwhile prevalence estimates of asthma among men and women ranged from 2.2% to 2.6% and 3.1% to 3.4%, respectively.
Table 1. Sociodemographic characteristics of men and women aged 20 years or over in Korean national health and nutrition examination survey (KNHANES) according to study period.
| Men (n = 20,180) | Women (n = 26,911) | |||||
|---|---|---|---|---|---|---|
| 2007–2009 | 2010–2012 | 2013–2015 | 2007–2009 | 2010–2012 | 2013–2015 | |
| All participants (Na) | 7,010 | 7,160 | 6,010 | 9,400 | 9,545 | 7,966 |
| Age, mean (SD) | 43.6 (0.27) | 44.4 (0.28) | 45.1 (0.26) | 45.4 (0.28) | 46.2 (0.26) | 46.7 (0.27) |
| Age group (years) | ||||||
| 20–29 | 887 (20.5) | 745 (19.3) | 719 (19.4) | 1,141 (18.7) | 1,068 (17.2) | 893 (16.4) |
| 30–39 | 1,412 (23.4) | 1,308 (21.9) | 978 (20.1) | 1,967 (21.8) | 1,871 (20.8) | 1,378 (19.4) |
| 40–49 | 1,445 (23.4) | 1,353 (23.0) | 1,112 (21.5) | 1,874 (22.5) | 1,717 (21.8) | 1,535 (21.7) |
| 50–59 | 1,213 (16.7) | 1,381 (18.7) | 1,195 (20.3) | 1,597 (16.2) | 1,930 (18.9) | 1,685 (20.5) |
| 60+ | 2,053 (16.0) | 2,373 (17.1) | 2,006 (18.7) | 2,821 (20.8) | 2,959 (21.3) | 2,475 (22.0) |
| Education | ||||||
| Elementary | 1,265 (11.7) | 1,049 (9.9) | 788 (8.7) | 3,139 (25.3) | 2,740 (22.7) | 1,920 (18.4) |
| Middle school | 905 (10.4) | 856 (9.6) | 682 (8.4) | 1,056 (10.9) | 1,091 (10.9) | 910 (9.9) |
| High school | 2,061 (30.6) | 2,144 (31.4) | 1,774 (29.4) | 2,707 (32.4) | 2,623 (30.4) | 2,263 (30.2) |
| University level or above | 2,779 (47.3) | 3,111 (49.1) | 2,766 (53.5) | 2,498 (31.4) | 3,091 (36.0) | 2,873 (41.5) |
| Diabetes | ||||||
| Yes | 725 (8.5) | 874 (9.1) | 820 (10.6) | 762 (6.9) | 789 (7.0) | 757 (7.8) |
| Arthritis | ||||||
| Yes | 398 (4.2) | 328 (3.0) | 323 (3.7) | 1,576 (13.2) | 1,584 (13.0) | 1,328 (12.9) |
| Asthma | ||||||
| Yes | 184 (2.2) | 207 (2.6) | 139 (2.3) | 340 (3.1) | 317 (3.1) | 286 (3.4) |
| Depressive symptoms | ||||||
| Yes | 737 (9.3) | 632 (9.0) | 343 (5.3) | 1,847 (18.6) | 1,550 (16.4) | 819 (10.2) |
| Marital status | ||||||
| Never married | 1,119 (23.3) | 704 (17.6) | 1,079 (26.7) | 976 (14.6) | 730 (11.4) | 991 (16.8) |
| Married/cohabiting | 5,453 (71.0) | 5,759 (70.0) | 4,542 (68.1) | 6,435 (67.6) | 6,785 (68.4) | 5,543 (68.7) |
| Divorced separated/ widowed | 402 (4.9) | 324 (4.2) | 380 (5.1) | 1,963 (17.4) | 1,640 (14.8) | 1,425 (14.4) |
| Missing | 36 (0.8) | 373 (8.2) | 9 (0.1) | 26 (0.4) | 390 (5.4) | 7 (0.1) |
| Smoking status | ||||||
| Never smoker | 1,278 (19.0) | 1,287 (19.5) | 1,304 (24.0) | 8,273 (86.5) | 8,532 (86.6) | 7,131 (88.3) |
| Former smoker | 2,638 (33.7) | 2,920 (33.8) | 2,404 (34.7) | 553 (6.7) | 504 (6.4) | 409 (5.7) |
| Current smoker | 3,093 (47.3) | 2,951 (46.7 | 2,281 (41.0) | 573 (6.8) | 509 (7.0) | 398 (5.7) |
| Missing | 1 (0.0) | 2 (0.0) | 21 (0.3) | 1 (0.0) | 0 (0.0) | 28 (0.3) |
| Alcohol consumption | ||||||
| No | 399 (4.8) | 350 (4.1) | 290 (4.0) | 2,011 (18.4) | 1,893 (15.9) | 1,374 (14.6) |
| Yes | 6,611 (95.2) | 6,809 (95.9) | 5,700 (95.7) | 7,387 (81.5) | 7,652 (84.1) | 6,572 (85.2) |
| Missing | 0 (0.0) | 1 (0.0) | 20 (0.3) | 2 (0.0) | 0 (0.0) | 20 (0.2) |
| Physical activity | ||||||
| No | 5,048 (73.2) | 5,627 (77.0) | 5,535 (91.6) | 7,205 (77.7) | 7,956 (83.3) | 7,552 (94.6) |
| Yes | 1,962 (26.8) | 1,533 (23.0) | 475 (8.4) | 2,195 (22.3) | 1,589 (16.7) | 414 (5.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Obesity | ||||||
| Underweight (< 18.5 kg/m2) | 247 (3.3) | 183 (2.4) | 139 (2.5) | 485 (6.2) | 528 (6.6) | 407 (6.3) |
| Normal (18.5 to 24.9 kg/m2) | 4,278 (59.9) | 4,418 (60.7) | 3,568 (59.0) | 6,098 (65.7) | 6,195 (64.9) | 5,258 (67.0) |
| Obese (>25 kg/m2) | 2,449 (36.3) | 2,538 (36.6) | 2,296 (38.4) | 2,777 (27.6) | 2,810 (28.4) | 2,296 (26.6) |
| Missing | 36 (0.5) | 21 (0.3) | 7 (0.1) | 40 (0.5) | 12 (0.1) | 5 (0.1) |
aUnless otherwise stated, results are expressed as unweighted frequencies with weighted percentages shown in brackets.
Age-standardised estimates of NCDs prevalence
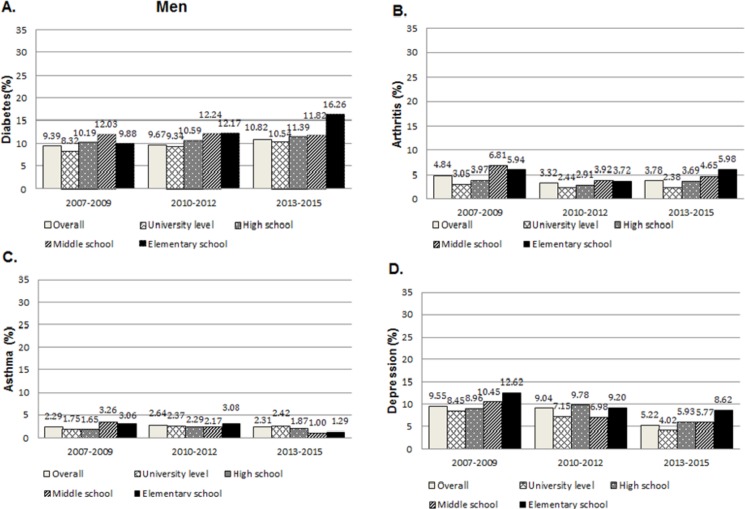
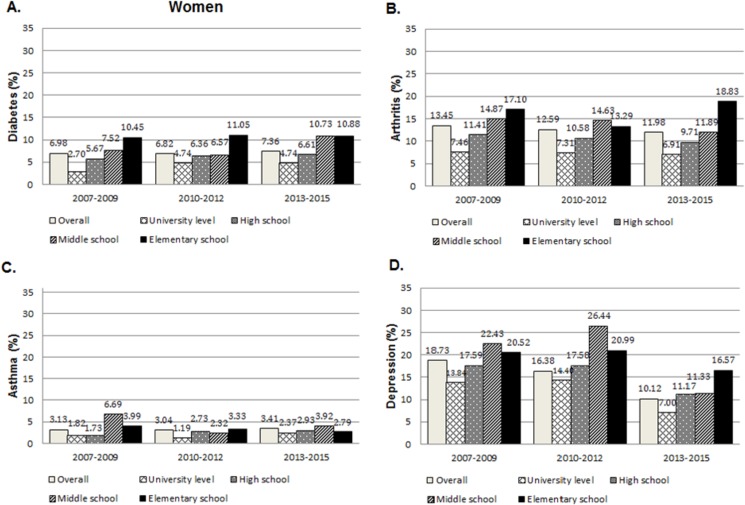
Figs 1 and 2 illustrate the age-standardised prevalence estimates (%) with 95% confidence intervals for each non-communicable disease by educational level. In men, the overall age-standardised prevalence of diabetes increased from 9.39 (95% CI 8.56 to 10.23) in 2007–2009 to 10.82% (95% CI 10.02 to 11.63) in 2013–2015 (Fig 1A). On the contrary, the overall prevalence of diabetes did not change significantly in women (Fig 2A). Prevalence of asthma remained relatively stable over the study period at around 2% and 3% for men and women, respectively. In general, there were downward trends with respect to arthritis and depressive symptoms, with overall prevalence decreasing in both men and women. For most NCDs, prevalence estimates generally declined with increasing educational level. The clearest pattern of relationship was seen among women and in 2013–2015. However, no consistent pattern was seen in the prevalence of asthma across the survey period.
Fig 1. Age-standardised prevalence and 95% CI of NCDs in men aged 20 or over, according to educational level–KNHANES between 2007–2015.
Fig 2. Age-standardised prevalence and 95% CI of NCDs in women aged 20 or over, according to educational level–KNHANES between 2007–2015.
Tables 2 and 3 present the prevalence ratios and their confidence intervals for the associations between level of educational attainment and NCDs. There has no significant association between diabetes and education among men. Women, on the other hand, showed a higher prevalence of diabetes in individuals with elementary education than their more educated counterparts, with age-adjusted PRs ranging from 1.99 to 4.90 (p for trend <0.001). Our data indicated a clear inverse gradient in prevalence of arthritis across educational groups for both genders, with higher prevalence amongst the subjects with lower educational attainment. These associations were significant for all survey years. For example, relative to those with university level education or above, the age-adjusted PRs of arthritis for the elementary education group in 2013–2015 was 2.74 (95% CI 2.05 to 3.66; p trend<0.001) among women and 2.60 (95% CI 1.70 to 3.98; p for trend <0.001) among men. Moreover, the results were generally robust to adjustments for smoking status, alcohol consumption, physical activity and obesity, although slight attenuations of the association were observed (Table 2, Model 2). The estimated prevalence ratios for asthma tended to be greater among the lowest education group at different time points, although some exceptions were noted. For example, in men, Poisson regression revealed non-significant association of education with asthma in 2013–2015.
Table 2. Prevalence ratios and 95% confidence interval for NCDs among men, by educational level and survey year from 2007 to 2015.
| 2007–2009 | 2010–2012 | 2013- 2015 |
||||
|---|---|---|---|---|---|---|
| Model 1† | Model 2‡ | Model 1† | Model 2‡ | Model 1† | Model 2‡ | |
| Diabetes | ||||||
| Elementary school | 1.11 (0.81 to 1.53) |
1.15 (0.84 to 1.60) |
1.14 (0.86 to 1.50) |
1.11 (0.84 to 1.47) |
1.14 (0.89 to 1.45) |
1.11 (0.87 to 1.42) |
| Middle school | 1.38 (1.02 to 1.88) |
1.40 (1.03 to 1.91) |
1.45 (1.09 to 1.91) |
1.39 (1.05 to 1.83) |
1.19 (0.92 to 1.55) |
1.16 (0.90 to 1.52) |
| High school | 1.32 (1.04 to 1.66) |
1.30 (1.03 to 1.65) |
1.41 (1.13 to 1.75) |
1.37 (1.09 to 171) |
1.28 (1.05 to 1.55) |
1.24 (1.02 to 1.50) |
| University level or above | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| p for trend | 0.478 | 0.331 | 0.298 | 0.396 | 0.331 | 0.438 |
| Arthritis | ||||||
| Elementary school | 2.70 (1.75 to 4.16) |
2.77 (1.78 to 4.31) |
1.52 (1.03 to 2.23) |
1.57 (1.07 to 2.33) |
2.60 (1.70 to 3.98) |
2.62 (1.71 to 4.04) |
| Middle school | 2.23 (1.47 to 3.43) |
2.22 (1.47 to 3.48) |
1.75 (1.14 to 2.69) |
1.76 (1.15 to 2.71) |
2.31 (1.42 to 3.76) |
2.32 (1.41 to 3.78) |
| High school | 1.52 (0.93 to 2.47) |
1.51 (0.92 to 2.47) |
1.15 (0.74 to 1.76) |
1.17 (0.76 to 1.80) |
1.85 (1.22 to 2.79) |
1.85 (1.22 to 2.81) |
| University level or above | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| p for trend | <0.001 | <0.001 | 0.010 | 0.006 | <0.001 | <0.001 |
| Asthma | ||||||
| Elementary school | 2.14 (1.22 to 3.77) |
2.22 (1.28 to 4.06) |
2.47 (1.35 to 4.52) |
2.43 (1.32 to 4.45) |
1.17 (0.58 to 2.37) |
1.21 (0.59 to 2.46) |
| Middle school | 1.45 (0.88 to 2.38) |
1.56 (0.92 to 2.58) |
1.47 (0.75 to 2.88) |
1.47 (0.75 to 2.89) |
0.97 (0.53 to 1.76) |
0.98 (0.54 to 1.80) |
| High school | 0.78 (0.49 to 1.26) |
0.82 (0.51 to 1.34) |
0.81 (0.49 to 1.32) |
0.81 (0.50 to 1.32) |
0.68 (0.40 to 1.14) |
0.69 (0.41 to 1.17) |
| University level or above | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| p for trend | 0.007 | 0.004 | 0.016 | 0.018 | 0.947 | 0.958 |
| Depressive symptoms | ||||||
| Elementary school | 1.79 (1.32 to 2.43) |
1.74 (1.28 to 2.35) |
1.87 (1.34 to 2.62) |
1.75 (1.25 to 2.45) |
2.78 (1.83 to 4.20) |
2.75 (1.82 to 4.18) |
| Middle school | 1.30 (0.96 to 1.77) |
1.25 (0.93 to 1.70) |
1.81 (1.32 to 2.46) |
1.73 (1.27 to 2.37) |
2.25 (1.43 to 3.56) |
2.25 (1.44 to 3.56) |
| High school | 1.12 (0.90 to 1.40) |
1.08 (0.86 to 1.35) |
1.36 (1.07 to 1.75) |
1.30 (1.02 to 1.67) |
1.58 (1.17 to 2.13) |
1.56 (1.15 to 2.11) |
| University level or above | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| p for trend | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 |
†Model 1: Adjusted for age.
‡Model 2: Adjusted for age, smoking status, alcohol consumption, physical activity and obesity.
Table 3. Prevalence ratios and 95% confidence interval for NCDs among women, by educational level and survey year from 2007 to 2015.
| 2007–2009 | 2010–2012 | 2013- 2015 |
||||
|---|---|---|---|---|---|---|
| Model 1† | Model 2‡ | Model 1† | Model 2‡ | Model 1† | Model 2‡ | |
| Diabetes | ||||||
| Elementary school | 4.90 (2.94 to 8.19) |
4.41 (2.69 to 7.23) |
3.75 (2.29 to 6.13) |
3.35 (2.07 to 5.42) |
1.99 (1.39 to 2.86) |
1.80 (1.26 to 2.58) |
| Middle school | 3.88 (2.37 to 6.33) |
3.56 (2.19 to 5.77) |
3.48 (2.19 to 5.54) |
3.19 (2.02 to 5.05) |
2.11 (1.48 to 3.01) |
1.93 (1.35 to 2.76) |
| High school | 2.75 (1.76 to 4.29) |
2.62 (1.68 to 4.07) |
2.73 (1.79 to 4.16) |
2.56 (1.68 to 3.89) |
1.58 (1.16 to 2.14) |
1.51 (1.10 to 2.05) |
| University level or above | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 |
| Arthritis | ||||||
| Elementary school | 3.45 (2.38 to 4.99) |
3.20 (2.24 to 4.57) |
3.68 (2.59 to 5.24) |
3.33 (2.35 to 4.70) |
2.74 (2.05 to 3.66) |
2.57 (1.93 to 3.41) |
| Middle school | 3.64 (3.54 to 5.22) |
3.39 (2.38 to 4.83) |
4.11 (2.95 to 5.74) |
3.74 (2.69 to 5.19) |
2.60 (1.96 to 3.46) |
2.41 (1.81 to 3.21) |
| High school | 1.83 (1.31 to 2.54) |
1.77 (1.27 to 2.46) |
2.24 (1.65 to 3.05) |
2.09 (1.54 to 2.85) |
1.74 (1.33 to 2.26) |
1.69 (1.30 to 2.20) |
| University level or above | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Asthma | ||||||
| Elementary school | 2.28 (1.26 to 4.14) |
2.08 (1.15 to 3.74) |
2.16 (1.26 to 3.69) |
2.10 (1.22 to 3.62) |
2.15 (1.47 to 3.16) |
2.06 (1.39 to 3.04) |
| Middle school | 1.46 (0.76 to 2.79) |
1.36 (0.72 to 2.58) |
1.55 (0.92 to 2.60) |
1.51 (0.89 to 2.58) |
1.34 (0.79 to 2.27) |
1.30 (0.77 to 2.18) |
| High school | 0.76 (0.48 to 1.20) |
0.70 (0.44 to 1.12) |
1.13 (0.72 to 1.76) |
1.10 (0.70 to 1.73) |
0.87 (0.58 to 1.31) |
0.85 (0.57 to 1.27) |
| University level or above | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| p for trend | 0.002 | 0.004 | 0.004 | 0.005 | <0.001 | <0.001 |
| Depressive symptoms | ||||||
| Elementary school | 1.95 (1.55 to 2.45) |
1.89 (1.50 to 2.37) |
1.81 (1.44 to 2.27) |
1.67 (1.34 to 2.08) |
2.95 (2.19 to 3.98) |
2.75 (2.04 to 3.70) |
| Middle school | 1.63 (1.34 to 1.98) |
1.61 (1.32 to 1.97) |
1.63 (1.31 to 2.01) |
1.51 (1.23 to 1.86) |
1.89 (1.39 to 2.56) |
1.82 (1.34 to 2.47) |
| High school | 1.24 (1.04 to 1.48) |
1.20 (1.01 to 1.42) |
1.24 (1.06 to 1.46) |
1.17 (0.99 to 1.38) |
1.49 (1.20 to 1.87) |
1.42 (1.14 to 1.77) |
| University level or above | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
†Model 1: Adjusted for age.
‡Model 2: Adjusted for age, smoking status, alcohol consumption, physical activity and obesity.
Relative index of inequality
Time trends in relative inequalities in NCDs are summarized in Table 4. Among women, the inequality in diabetes between educational levels appeared to decrease over time. The RII value decreased from 7.59 (4.04 to 14.22) in 2007–2009 to 2.58 (1.61 to 4.15) in 2013–2015 (Table 4), however, the test for overall trend in RII was not statistically significant (p = 0.096). Regarding gender differences, relative inequalities in diabetes were more marked among women than in men in all surveys (All P for gender interaction: p<0.001; see Table 5). For arthritis, the RII showed a consistent tendency towards reduced inequalities from 2007–2009 to 2013–2015 among women (p = 0.033) but not in men (p = 0.227). Furthermore, there was a significant ridit-score by gender interaction in 2010–2012 (P for gender interaction = 0.035). In relation to asthma, narrowing of educational inequalities was noted for both men and women, with significant differences in RII observed among males and females in 2010–2012 and 2013–2015. For depressive symptoms, RII showed a non-significant trend towards widening educational disparities in depressive symptoms (p for trend 0.156 in men and 0.881 in women) and no significant gender differences in RII were noted (all P values >0.05).
Table 4. Trends in educational inequalities in NCDs between 2007 and 2015.
| Men | 2007–2009 | 2010–2012 | 2013–2015 | P for trend across surveys† |
| RII (95% CI) | RII (95% CI) | RII (95% CI) | ||
| Diabetes | 1.37 (0.90 to 2.08) | 1.46 (1.02 to 2.11)* | 1.30 (0.94 to 1.80) | 0.758 |
| Arthritis | 4.09 (2.21 to 7.62)*** | 2.01 (1.14 to 3.56)** | 3.89 (2.15 to 7.07)*** | 0.227 |
| Asthma | 2.00 (0.94 to 4.26) | 1.74 (0.76 to 4.00) | 0.75 (0.33 to 1.69) | 0.001 |
| Depressive symptoms | 1.81 (1.21 to 2.72)*** | 2.42 (1.57 to 3.75)*** | 3.82 (2.17 to 6.73)*** | 0.156 |
| Women | ||||
| Diabetes | 7.59 (4.04 to 14.22)*** | 5.10 (2.84 to 9.18)*** | 2.58 (1.61 to 4.15)*** | 0.096 |
| Arthritis | 5.79 (3.52 to 9.54)*** | 5.46 (3.57 to 8.36)*** | 3.98 (2.74 to 5.79)*** | 0.033 |
| Asthma | 3.17 (1.24 to 8.09)** | 2.82 (1.30 to 6.09)** | 2.51 (1.43 to 4.41)** | 0.047 |
| Depressive symptoms | 2.50 (1.83 to 3.43)*** | 2.29 (1.67 to 3.14)*** | 4.24 (2.77 to 6.49)*** | 0.881 |
RII values larger than 1 indicate that the prevalence of NCDs is higher in the less educated group.
†P-values for the interactions between survey period and ridit-score.
* p<0.05.
**p<0.01.
*** p<0.001.
Table 5. Gender differences in relative index of inequality in NCDs.
| 2007–2009 | 2010–2012 | 2013–2015 | |
|---|---|---|---|
| Diabetes | 1.83 (1.31 to 2.34) | 1.36 (0.92 to 1.80) | 1.06 (0.69 to 1.43) |
| <0.001* | <0.001* | <0.001* | |
| Arthritis | 1.73 (1.26 to 2.19) | 1.60 (1.20 to 2.01) | 1.37 (1.01 to 1.72) |
| p = 0.416* | p = 0.035* | p = 0.925* | |
| Asthma | 1.12 (0.36 to 1.88) | 1.54 (0.81 to 2.27) | 1.17 (0.58 to 1.77) |
| p = 0.437* | p = 0.003* | p<0.001* | |
| Depressive symptoms | 0.85 (0.57 to 1.13) | 0.77 (0.49 to 1.05) | 1.38 (0.98 to 1.79) |
| p = 0.343* | p = 0.406* | p = 0.913* |
* Results from the two-way interaction term—Ridit score* gender.
Discussion
Using a representative sample of Korean adult population obtained by repeated cross-sectional surveys, we examined the trends in social distribution of diabetes, arthritis, asthma and depressive symptoms, among Korean adults between 2007 and 2015. A higher level of education was generally associated with more favorable outcomes; an exception was diabetes among men where no clear association with educational attainment was observed. This is in agreement with results reported by Imkampe and Gulliford who in the analyses of four repeated cross-sectional surveys in England between 1994 and 2006 found no significant relationship between educational level and diabetes men, but increasing relative inequalities women [36]. As has been shown by our study, numerous studies has documented inverse socioeconomic gradients in the incidence and prevalence of diabetes [37–40], arthritis [41], asthma [42, 43], and depression [44–46]. One common explanation given for the observed inverse educational gradient in NCDs is the variation in established risk factors of NCDs such as hypertension [47], obesity [48], smoking [49] and poorer dietary intake [50, 51], by educational level. Limited access to high-quality healthcare services among the less advantaged groups may also contribute to the inequalities in NCDs. In our data, however, the effect of education on NCDs remained significant after adjustment for age and multiple potentially mediating behavioral risk factors, suggesting that education as a risk factor for NCDs is at least partially independent of these covariates. In terms of secular trends, we observed trends of widening disparities in depressive symptoms over time, while inequalities have narrowed for diabetes, arthritis and asthma. Although there are no strictly comparable Korean studies on trends in educational inequalities in NCDs, our results are in accordance with previous research conducted by Hong et al. that demonstrated a widening pro-rich inequality in depression, which doubled over a 10 year period from 1997 to 2007 [52].
Another notable finding of the study is the significant gender differences in social patterning of NCDs. This corresponds with previous work demonstrating a steeper education gradient among women relative to men [53, 54]. The exact reasons for the observed gender heterogeneity remain uncertain. However, differences in exposure and vulnerability to material and behavioural risk factors have been postulated as possible explanations for the gender differences in social pattering of diabetes [55]. For example, it is well known that obesity, an important determinant of diabetes, is particularly prevalent among women with lower socioeconomic circumstances. Another likely explanation involves differentiated social roles, variations in exposure to stressors and coping strategies between men and women [56, 57]. Research has indicated that psychosocial stressors play an important role in determining health, but their effects are generally stronger for women relative to men. Also, women are typically are more involved in familial nurturing roles, while men’s roles are more instrumental. Thus, women are more likely to exposed to chronic stressors, which may in turn, be related to increased risk of developing NCDs. These explanations however remain speculative and merits further research.
There are a number of methodological limitations that limit the generalizability of our findings. First, the use of self-reported measures may have introduced recall error. For instance, the assessment of NCDs were based on self-reports, potentially leading to misclassification of disease status. Nonetheless, self-reported measures are frequently used in many population surveys for the surveillance of chronic diseases. Second, the sample sizes of the KNHANES were relatively small, especially after stratification by gender. Given the wide confidence intervals for the estimated RIIs, one must be cautious in generalizing the results. Further studies would benefit from a larger sample size to obtain more precise estimates of the trends in inequalities in NCDs and confirm the generalizability of our findings. A further limitation of this work is the possibility of selection bias due to the fall in response rates of KNHAES surveys in recent years [23]. Also, there is a potential bias through the decision to exclude those with missing information on education from our study. Since the analyzed participants were more likely to belong to the higher educational groups and were slightly younger than those excluded, we may have underestimated the prevalence of NCDs and possibly biased our results towards an underestimation of the association between NCDs and education. However, our estimate of self-reported NCD prevalence are comparable with reports using other sources of data [58, 59]. For example, according to the Korean National Health Insurance Service database (NHIS), diabetes prevalence for adults 30 years or older in 2012 was reported to be 7.1% and 8.5% for men and women, respectively. Despite these limitations, the present study also has several strengths. First, the use of a nationally representative sample surveyed over a 9-year period provided a comprehensive picture of the temporal trends in educational inequalities in non-communicable diseases among Korean adults. Second, because education is usually completed by early adulthood prior to the onset of chronic diseases, using educational attainment as a proxy for socioeconomic status has the advantage of minimizing the likelihood of reverse causality. Further, all surveys have been conducted according to standardised measurement protocol, which facilitated comparisons of social distribution of NCDs between different time points. To the best of our knowledge, this is one of very few studies examining the social patterning of non-communicable diseases in Korea. There is a need for prospective population-based studies designed to investigate underlying causes of these inequalities. This would improve the understanding of the development of inequalities in non-communicable diseases and could be beneficial for designing effective interventions strategies for Korean adults.
Conclusions
In summary, the results of this study demonstrated variations in the association of non-communicable diseases and SEP by gender with a tendency for an inverse social gradient among Korean adults. Whilst educational inequalities in depressive symptoms remained relatively stable, educational differences in diabetes, arthritis, and asthma have generally narrowed over 9 years since 2007. These findings highlight the importance of gender-based population level interventions aimed at preventing NCDs.
Data Availability
Data are available from the Korean National Health and Nutrition Examination Survey database. (URL: https://knhanes.cdc.go.kr/knhanes/eng/sub03/sub03_02_02.do).
Funding Statement
The authors have no funding or support to report.
References
- 1.World Health Organization WHO. World Health Statistics 2017: Monitoring Health for the SDGs. Geneva: World Health Organization, 2017. 2017. [Google Scholar]
- 2.Low W-Y, Lee Y-K, Samy AL. Non-communicable diseases in the Asia-Pacific region: Prevalence, risk factors and community-based prevention. International journal of occupational medicine and environmental health. 2015:1–7. doi: 10.13075/ijomeh.1896.00603 [DOI] [PubMed] [Google Scholar]
- 3.Yoon J, Oh I-H, Seo H, Kim E-J, Gong Y-h, Ock M, et al. Disability-adjusted life years for 313 diseases and injuries: the 2012 Korean burden of disease study. Journal of Korean medical science. 2016;31(Suppl 2):S146–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon J, Seo H, Oh I-H, Yoon S-J. The Non-Communicable Disease Burden in Korea: Findings from the 2012 Korean Burden of Disease Study. Journal of Korean medical science. 2016;31(Suppl 2):S158–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H-Y, Kim J. Trends of Income Inequality in Korea Green and Smart Technology with Sensor Applications: Springer; 2012. p. 1–12. [Google Scholar]
- 6.Lee H, Lee M, Park D. Growth policy and inequality in developing Asia: Lesson from Korea. ERIA Discussion Paper Series, http://eria.org/ERIA-DP-2012-12.pdf. 2012.
- 7.Suh M, Kim H, Suh T, Sun W, Shin Y, Choi E. Establish of New Health Plan 2010. Seoul: Korea Institute for Health and Social Affairs, Ministry of Health and Welfare. 2005. [Google Scholar]
- 8.Sommer I, Griebler U, Mahlknecht P, Thaler K, Bouskill K, Gartlehner G, et al. Socioeconomic inequalities in non-communicable diseases and their risk factors: an overview of systematic reviews. BMC public health. 2015;15(1):914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee TC, Glynn RJ, Peña JM, Paynter NP, Conen D, Ridker PM, et al. Socioeconomic status and incident type 2 diabetes mellitus: data from the Women's Health Study. PLoS One. 2011;6(12):e27670 doi: 10.1371/journal.pone.0027670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer causes & control. 2009;20(4):417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershon AS, Dolmage TE, Stephenson A, Jackson B. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2012;9(3):216–26. doi: 10.3109/15412555.2011.648030 [DOI] [PubMed] [Google Scholar]
- 12.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4):1973–98. [DOI] [PubMed] [Google Scholar]
- 13.Khang Y-H, Lynch J, Yun S, Lee S. Trends in socioeconomic health inequalities in Korea: use of mortality and morbidity measures. Journal of Epidemiology and Community Health. 2004;58(4):308–14. doi: 10.1136/jech.2003.012989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim M-H, Jung-Choi K, Kim H, Song Y-M. Educational inequality in female cancer mortality in Korea. Journal of Korean medical science. 2015;30(1):1–6. doi: 10.3346/jkms.2015.30.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M-H, Jung-Choi K, Ko H, Song Y-M. Educational Inequality in Obesity-Related Mortality in Korea. Journal of Korean medical science. 2017;32(3):386–92. doi: 10.3346/jkms.2017.32.3.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min JW. Trends in income-related health inequalities in self-assessed health in Korea, 1998–2011. Global public health. 2014;9(9):1053–66. doi: 10.1080/17441692.2014.931448 [DOI] [PubMed] [Google Scholar]
- 17.Chun H, Cho S-I, Khang Y-H, Kang M, Kim I-H. Trends in gender-based health inequality in a transitional society: a historical analysis of South Korea. Journal of Preventive Medicine and Public Health. 2012;45(2):113–21. doi: 10.3961/jpmph.2012.45.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J-M, Kim H-M, Jung B-Y, Park E-C, Cho W-H, Lee S-G. The association between cancer incidence and family income: analysis of Korean National Health Insurance cancer registration data. Asian Pacific Journal of Cancer Prevention. 2012;13(4):1371–6. [DOI] [PubMed] [Google Scholar]
- 19.Choi S-W, Ryu S-Y, Han M-a, Park J. The association between the socioeconomic status and thyroid cancer prevalence; based on the Korean National Health and Nutrition Examination Survey 2010–2011. Journal of Korean medical science. 2013;28(12):1734–40. doi: 10.3346/jkms.2013.28.12.1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo SR, Kim SY, Lee S-Y, Yoon T-H, Park H-G, Lee SE, et al. The incidence of stroke by socioeconomic status, age, sex, and stroke subtype: a nationwide study in Korea. Journal of Preventive Medicine and Public Health. 2014;47(2):104 doi: 10.3961/jpmph.2014.47.2.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ock M, Han JW, Lee JY, Kim S-H, Jo M-W. Estimating quality-adjusted life-year loss due to noncommunicable diseases in Korean adults through to the year 2040. Value in Health. 2015;18(1):61–6. doi: 10.1016/j.jval.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 22.Park HA. The Korea national health and nutrition examination survey as a primary data source. Korean journal of family medicine. 2013;34(2):79–. doi: 10.4082/kjfm.2013.34.2.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kweon S, Kim Y, Jang M-j, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea national health and nutrition examination survey (KNHANES). International journal of epidemiology. 2014;43(1):69–77. doi: 10.1093/ije/dyt228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H-J, Ruger JP. Socioeconomic disparities in behavioral risk factors and health outcomes by gender in the Republic of Korea. BMC public health. 2010;10(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon TH, Moon OR, Lee SY, Jeong BG, Lee SJ, Kim NS, et al. Differences in health behaviors among the social strata in Korea. Korean Journal of Preventive Medicine. 2000;33(4):469–76. [Google Scholar]
- 26.Khang Y-H, Cho H-J. Socioeconomic inequality in cigarette smoking: trends by gender, age, and socioeconomic position in South Korea, 1989–2003. Preventive medicine. 2006;42(6):415–22. doi: 10.1016/j.ypmed.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 27.Organization WH. WHO: Steering Committee of the Western Pacific Region of the WHO, the International Association for the Study of Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. 2000. [Google Scholar]
- 28.Lee D, Kim Y, Han H. Sex differences in the association between socio-economic status and type 2 diabetes: data from the 2005 Korean National Health and Nutritional Examination Survey (KNHANES). Public Health. 2013;127(6):554–60. doi: 10.1016/j.puhe.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 29.Eachus J, Williams M, Chan P, Smith GD, Grainge M, Donovan J, et al. Deprivation and cause specific morbidity: evidence from the Somerset and Avon survey of health. Bmj. 1996;312(7026):287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller G, Hartwig S, Greiser KH, Moebus S, Pundt N, Schipf S, et al. Gender differences in the association of individual social class and neighbourhood unemployment rate with prevalent type 2 diabetes mellitus: a cross-sectional study from the DIAB-CORE consortium. BMJ open. 2013;3(6):e002601 doi: 10.1136/bmjopen-2013-002601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC medical research methodology. 2003;3(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Chia K. Prevalence odds ratio v prevalence ratio—a response. Occupational and environmental medicine. 1995;52(11):781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Localio AR, Margolis DJ, Berlin JA. Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. Journal of clinical epidemiology. 2007;60(9):874–82. doi: 10.1016/j.jclinepi.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 35.Kunst AE, Mackenbach JP. The size of mortality differences associated with educational level in nine industrialized countries. American journal of public health. 1994;84(6):932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imkampe AK, Gulliford MC. Increasing socio-economic inequality in type 2 diabetes prevalence—repeated cross-sectional surveys in England 1994–2006. The European Journal of Public Health. 2011;21(4):484–90. doi: 10.1093/eurpub/ckq106 [DOI] [PubMed] [Google Scholar]
- 37.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. International journal of epidemiology. 2011:dyr029. [DOI] [PubMed] [Google Scholar]
- 38.Lidfeldt J, Li TY, Hu FB, Manson JE, Kawachi I. A prospective study of childhood and adult socioeconomic status and incidence of type 2 diabetes in women. American Journal of Epidemiology. 2007;165(8):882–9. doi: 10.1093/aje/kwk078 [DOI] [PubMed] [Google Scholar]
- 39.Borrell LN, Dallo FJ, White K. Education and diabetes in a racially and ethnically diverse population. American journal of public health. 2006;96(9):1637–42. doi: 10.2105/AJPH.2005.072884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malta DC, Bernal RTI, de Souza MdFM, Szwarcwald CL, Lima MG, de Azevedo Barros MB. Social inequalities in the prevalence of self-reported chronic non-communicable diseases in Brazil: national health survey 2013. International Journal for Equity in Health. 2016;15(1):153 doi: 10.1186/s12939-016-0427-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalstra JA, Kunst AE, Borrell C, Breeze E, Cambois E, Costa G, et al. Socioeconomic differences in the prevalence of common chronic diseases: an overview of eight European countries. International journal of epidemiology. 2005;34(2):316–26. doi: 10.1093/ije/dyh386 [DOI] [PubMed] [Google Scholar]
- 42.Basagaña X, Sunyer J, Kogevinas M, Zock J-P, Duran-Tauleria E, Jarvis D, et al. Socioeconomic status and asthma prevalence in young adults the European community respiratory health survey. American Journal of Epidemiology. 2004;160(2):178–88. doi: 10.1093/aje/kwh186 [DOI] [PubMed] [Google Scholar]
- 43.Eagan TM, Gulsvik A, Eide GE, Bakke PS. The effect of educational level on the incidence of asthma and respiratory symptoms. Respiratory medicine. 2004;98(8):730–6. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura J. Socioeconomic status and depression across Japan, Korea, and China: Exploring the impact of labor market structures. Social Science & Medicine. 2011;73(4):604–14. [DOI] [PubMed] [Google Scholar]
- 45.Lorant V, Deliège D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis Oxford Univ Press; 2003. [DOI] [PubMed] [Google Scholar]
- 46.Bjelland I, Krokstad S, Mykletun A, Dahl AA, Tell GS, Tambs K. Does a higher educational level protect against anxiety and depression? The HUNT study. Social science & medicine. 2008;66(6):1334–45. [DOI] [PubMed] [Google Scholar]
- 47.Scholes S, Bajekal M, Love H, Hawkins N, Raine R, O'Flaherty M, et al. Persistent socioeconomic inequalities in cardiovascular risk factors in England over 1994–2008: a time-trend analysis of repeated cross-sectional data. BMC public health. 2012;12(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon YS, Oh SW, Park HS. Socioeconomic status in relation to obesity and abdominal obesity in Korean adults: a focus on sex differences. Obesity. 2006;14(5):909–19. doi: 10.1038/oby.2006.105 [DOI] [PubMed] [Google Scholar]
- 49.Hanibuchi T, Nakaya T, Honjo K. Trends in socioeconomic inequalities in self-rated health, smoking, and physical activity of Japanese adults from 2000 to 2010. SSM-Population Health. 2016;2:662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Irala-Estevez J, Groth M, Johansson L, Oltersdorf U. A systematic review of socio-economic differences in food habits in Europe: consumption of fruit and vegetables. European journal of clinical nutrition. 2000;54(9):706 [DOI] [PubMed] [Google Scholar]
- 51.Kim K, Hong SA, Kim MK. Trends in nutritional inequality by educational level: a case of South Korea. Nutrition. 2010;26(7):791–8. [DOI] [PubMed] [Google Scholar]
- 52.Hong J, Knapp M, McGuire A. Income‐related inequalities in the prevalence of depression and suicidal behaviour: a 10‐year trend following economic crisis. World Psychiatry. 2011;10(1):40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown K, Nevitte A, Szeto B, Nandi A. Growing social inequality in the prevalence of type 2 diabetes in Canada, 2004–2012. Can J Public Health. 2015;106(3):132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Espelt A, Borrell C, Palència L, Goday A, Spadea T, Gnavi R, et al. Socioeconomic inequalities in the incidence and prevalence of type 2 diabetes mellitus in Europe. Gaceta Sanitaria. 2013;27(6):494–501. doi: 10.1016/j.gaceta.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 55.McDonough P, Walters V. Gender and health: reassessing patterns and explanations. Social science & medicine. 2001;52(4):547–59. [DOI] [PubMed] [Google Scholar]
- 56.MacIntyre S, Hunt K. Socio-economic position, gender and health: How do they interact? Journal of Health Psychology. 1997;2(3):315–34. doi: 10.1177/135910539700200304 [DOI] [PubMed] [Google Scholar]
- 57.Jensen I, Nygren Å, Gamberale F, Goldie I, Westerholm P. Coping with long-term musculoskeletal pain and its consequences: is gender a factor? Pain. 1994;57(2):167–72. [DOI] [PubMed] [Google Scholar]
- 58.Noh J, Han K-D, Ko S-H, Ko KS, Park C-Y. Trends in the pervasiveness of type 2 diabetes, impaired fasting glucose and co-morbidities during an 8-year-follow-up of nationwide Korean population. Scientific Reports. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim B-K, Kim J-Y, Kang M-K, Yang M-S, Park H-W, Min K-U, et al. Allergies are still on the rise? A 6-year nationwide population-based study in Korea. Allergology International. 2016;65(2):186–91. doi: 10.1016/j.alit.2015.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Korean National Health and Nutrition Examination Survey database. (URL: https://knhanes.cdc.go.kr/knhanes/eng/sub03/sub03_02_02.do).