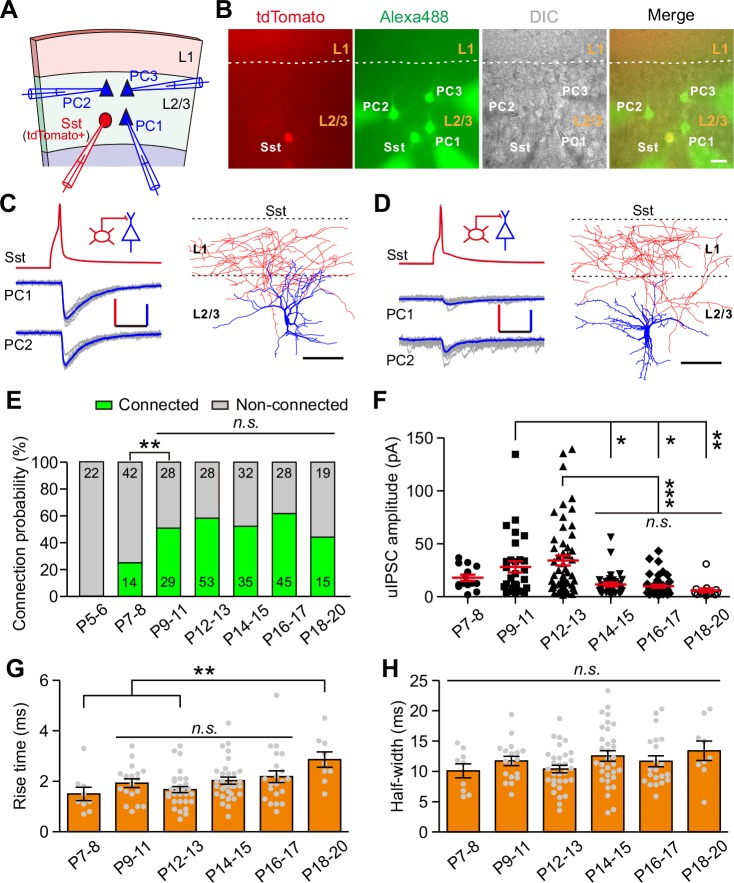
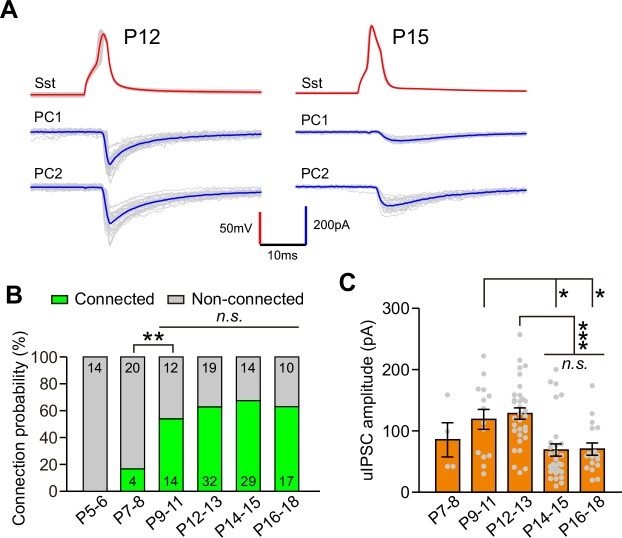
Figure 1. Development of synaptic transmission from Sst-INs onto PCs in layer 2/3 of the visual cortex.
(A) Schema of a quadruple whole-cell recording from an Sst-IN (red) and three PCs (blue) in layer 2/3. (B) Representative fluorescent (tdTomato, Sst-INs; Alexa 488, recorded neurons), IR-DIC, and merged images of a quadruple recording of an Sst-IN and three PCs. The dashed lines indicate the border between layer 1 and layer 2/3. Scale bar, 20 μm. (C) Left, representative traces showing synaptic transmission from an Sst-IN to two PCs recorded at P11. The red and blue lines indicate the averaged traces. Scale bars: 50 mV (vertical, red), 25 pA (vertical, blue), and 20 ms (horizontal). Right, morphological reconstruction of the Sst-IN. Scale bar: 80 µm. (D) Left, representative traces showing synaptic transmission from an Sst-IN to two PCs recorded at P15. Scale bars: 50 mV (vertical, red), 25 pA (vertical, blue), and 20 ms (horizontal). Right, morphological reconstruction of the Sst-IN. Scale bar: 80 µm. (E) The probability of synaptic connection from Sst-INs to PCs at P5–20. Data label indicates the number of pairs in each group. A total of 391 pairs were recorded from 82 mice. (F) Quantification of the peak amplitude of uIPSCs from Sst-INs to PCs at different postnatal ages. (G–H) Quantification of the 10–90% rise time (G) and half-width (H) of uIPSCs at P7–20. Detailed statistical analysis, detailed data, and exact sample numbers are presented in Figure 1—source data 1. Error bars indicate mean ±SEM. *p<0.05; **p<0.01; ***p<0.001; n.s., p>0.05.