Fig. 2.
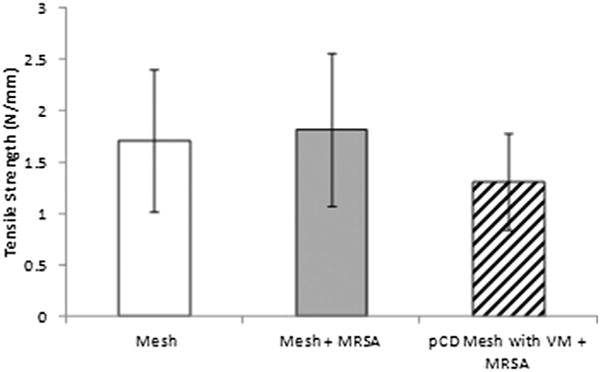
Mechanical testing (tissue integration strength) of samples at the study end point (30 d). Mechanical pull-strength testing was performed on all samples for the three conditions: (1) Uninfected control animals receiving normal mesh implants with no introduced infection; (2) infected control animals receiving normal mesh implants but receiving 106 CFU of MRSA; and (3) experimental conditions where animals receive antibiotic delivery mesh implants, as well as 106 CFU of MRSA. There was no significant difference between the three conditions (P = 0.15), and all tested samples had failure points at the mesh-tissue interface.
