Abstract
Hagfishes defend themselves from fish predators by releasing large volumes of gill-clogging slime when they are attacked. Slime release is not anticipatory, but is only released after an attack has been initiated, raising the question of how hagfishes survive the initial attack, especially from biting predators such as sharks. We tested two hypotheses that could explain how hagfishes avoid damage from shark bites: puncture-resistant skin, and a loose and flaccid body design that makes it difficult for teeth to penetrate body musculature and viscera. Based on data from skin puncture tests from 22 fish species, we found that hagfish skin is not remarkably puncture resistant. Simulated shark bites on hagfish and their closest living relatives, lamprey, as well as whole animal inflation tests, revealed that the loose attachment of hagfish skin to the rest of the body and the substantial ‘slack volume' in the subcutaneous sinus protect hagfish musculature and viscera from penetrating teeth. While recent work has found evidence that the capacious subcutaneous sinus in hagfishes is important for behaviours such as knot-tying and burrowing, our work demonstrates that it also plays a role in predator defence.
Keywords: hagfish, sharks, predator–prey interactions, puncture resistance
1. Introduction
Hagfishes are an ancient group of craniates found mostly in deep marine habitats [1]. Video footage of hagfishes being attacked in the wild [2], laboratory studies of slime function [3,4] and the conspicuous absence of hagfishes in the stomach contents of fishes [1] demonstrate that hagfish slime is an effective strategy for deterring fish predators. By quickly releasing slime after the onset of an attack, hagfishes make it difficult for fish to ingest them without fouling their gills and risking suffocation. In the case of suction feeding fishes, the slime provides an engulfed hagfish with a chance to escape as the predator reacts to the sliming of its gills. In the case of biting predators such as sharks, the slime appears to be equally effective at fouling the gills and discouraging the continuation of an attack [2]. However, because the slime is released after the hagfish is bitten, such a defence strategy is only effective if the hagfish can survive the initial bite. These observations raise the possibility that hagfishes possess adaptations that allow them to survive attacks by biting predators such as sharks.
We explored two possible protective mechanisms: puncture-resistant skin, and a flaccid and loose body design that minimizes injury by allowing the body to move out of the way of penetrating teeth. To test the first hypothesis, we measured the puncture resistance of hagfish skin and the skin of 21 other fish species representing a wide range of classes and subclasses. Hagfish skin is made up of three layers: epidermis, dermis and hypodermis [5] and differs from the skin of most gnathostome fishes in that it lacks scales. After accounting for a significant effect of skin thickness (p < 0.001), the load at puncture for Pacific hagfish (Eptatretus stoutii) skin was not significantly different from the skin of the other fish species tested (p = 0.930) (figure 1a). While these results rule out exceptional puncture resistance as an explanation for the ability of hagfishes to survive shark bites, the performance of hagfish skin is notable because hagfishes lack scales, which are known to boost puncture resistance in some cases by an order of magnitude [6]. How hagfish skin achieves this level of puncture resistance without scales is unknown and worthy of further study. While the force at puncture of hagfish skin was unremarkable, the extension at puncture was slightly higher (p = 0.012) than it was for other fish species after accounting for the significant effects (p < 0.001) of skin thickness (figure 1b). The higher extensibility of hagfish skin may contribute to the second protective mechanism we investigated.
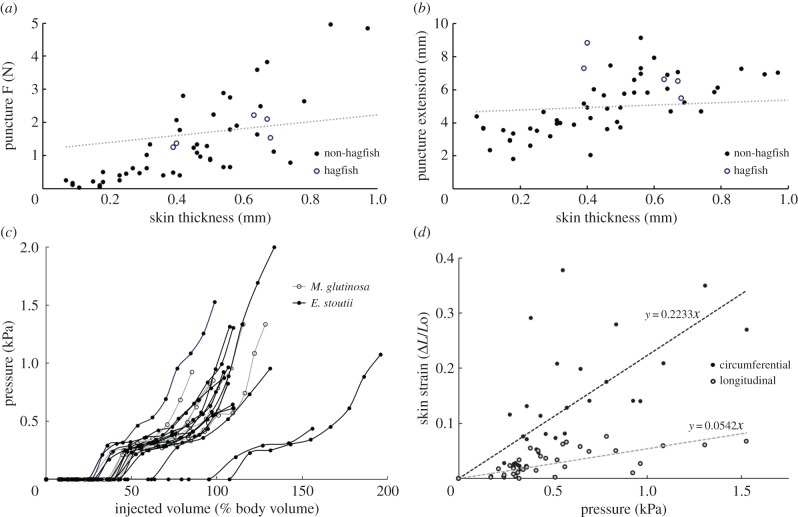
Figure 1.
Puncture and inflation of hagfish skin. (a) Load at puncture for E. stoutii skin did not differ significantly (p = 0.930) from values from 21 other fish species when the significant covariate of skin thickness (p < 0.001) was considered, whereas extension at puncture (b) was slightly and significantly higher (p = 0.012). (c) Injection of hagfish Ringer's solution into the SCS and simultaneous measurement of hydrostatic pressure resulted in pressure–volume curves for 13 E. stoutii and six M. glutinosa specimens. The flat part of the inflation curve before pressure starts to rise provides a quantitative measure of an animal's flaccidity or ‘slack volume', which in both species was substantial relative to the animal's volume, but did not differ between species (p = 0.33). (d) Skin strain in the longitudinal and circumferential directions as a function of inflation pressure. At higher pressures, circumferential strain was 4.5 times higher than longitudinal strain, suggesting stiffer mechanical properties of the skin in the longitudinal direction.
The second hypothesis we tested is that the loose and flaccid body design of hagfishes confers protection against shark bites. The hagfish body plan includes a large subcutaneous sinus (SCS) that runs the length of their body and contains 30% of the blood volume [7]. Consequently, a hagfish's body is only attached to the skin at the dorsal midline and at the slime glands [8]. Furthermore, hagfish skin is flaccid, meaning it can accommodate more volume than the body and the blood in the SCS occupy. The flaccidity of the skin was recently shown to have consequences for the ability of hagfishes to squeeze through narrow openings, as it allows them to locally reduce their dimensions by leaving some of their blood behind [9]. In the case of protecting themselves against biting predators, flaccid skin may be necessary to give the unattached body room to move out of the way of penetrating teeth. Here, we tested three critical predictions of the loose and flaccid hagfish hypothesis: (i) hagfish skin is flaccid enough to allow the body musculature and viscera to move out of the way of a penetrating tooth. (ii) Increasing attachment of the skin and the body should reduce the protective effects against penetrating teeth. (iii) Reducing the flaccidity of the skin should reduce the protective effects from penetrating teeth.
To quantify the flaccidity of hagfish skin, we injected hagfish Ringer's solution into the SCS of freshly dead E. stoutii and Atlantic hagfish (Myxine glutinosa) and simultaneously measured the internal pressure using a single-column manometer. These experiments revealed that the SCS of both species of hagfishes can indeed accommodate large volumes of fluid before the internal pressure begins to rise, which we refer to as the ‘slack volume'. The average slack volume for E. stoutii, measured as a per cent of body volume, was 46 ± 7% and 36 ± 2% for M. glutinosa, a difference that was not statistically significant (p = 0.33) (figure 1c). The E. stoutii individuals we tested were more variable in their slack volumes, which may have been due to greater variability in their time in captivity and the time from their last feeding compared to the M. glutinosa specimens. It is worth noting that the SCS of both species of hagfishes was able to accommodate substantially more fluid as the internal pressure increased. At pressures approaching aortic blood pressure in hagfishes (i.e. approx. 1 kPa) [10], the SCS was able to accommodate approximately 100% of the animal's starting volume. The maximum volume of Ringer's that could be injected before leakage occurred, expressed as a percentage of body volume, was 120 ± 8% for Pacific hagfish and 105 ± 6% for the Atlantic hagfish, a difference that was not statistically significant (p = 0.13) (figure 1c). Inflation trials provided a unique opportunity to probe the mechanical behaviour of hagfish skin in different dimensions without the potentially confounding effects of dissecting samples from the body. Laplace's law states that inflation of a cylindrical and isotropic skin should generate twice as much strain in the circumferential as the longitudinal direction [11]. We found a 4.5-fold difference in the two strain directions (figure 1d), which suggests that hagfish skin is anisotropic, with greater stiffness in the longitudinal axis, a result that is consistent with recent findings by Clark et al. [12].
To observe interactions between hagfishes and shark teeth in a controlled setting, we constructed a custom spring-driven guillotine (electronic supplementary material, figure S1) that allowed us to drive mako shark (Isurus sp.) teeth into freshly dead hagfish from both species at speeds comparable to real shark attacks. This device also allowed us to test the effects of skin flaccidity and the degree of attachment between the skin and body (figure 2 and table 1). In both species of hagfishes tested in the guillotine in the natural state, the skin was punctured 100% of the time, but the underlying parietal muscle was never damaged. When skin flaccidity was reduced by dissecting away a continuous cylinder of skin, moving it to a part of the body with a larger girth, and pinning it tight, the skin was punctured nearly 100% of the time, and the underlying muscle was punctured almost every time. To measure the effect of skin attachment, we glued the skin to the underlying muscle using dots of cyanoacrylate glue, and found that the skin was always punctured and the parietal muscle was punctured in 92% of the trials. To test whether it was the adhesion between the skin and body or the mere presence of the glue that caused the increase in damage seen in the glued trials, we conducted trials in which glue was applied to the underlying muscle and allowed to cure before the skin was replaced. In these trials, the skin was punctured in most trials, but the parietal muscle was rarely damaged, suggesting that it was the adhesion itself, and not the presence of the glue that increased the risk of damage in the glued trials.
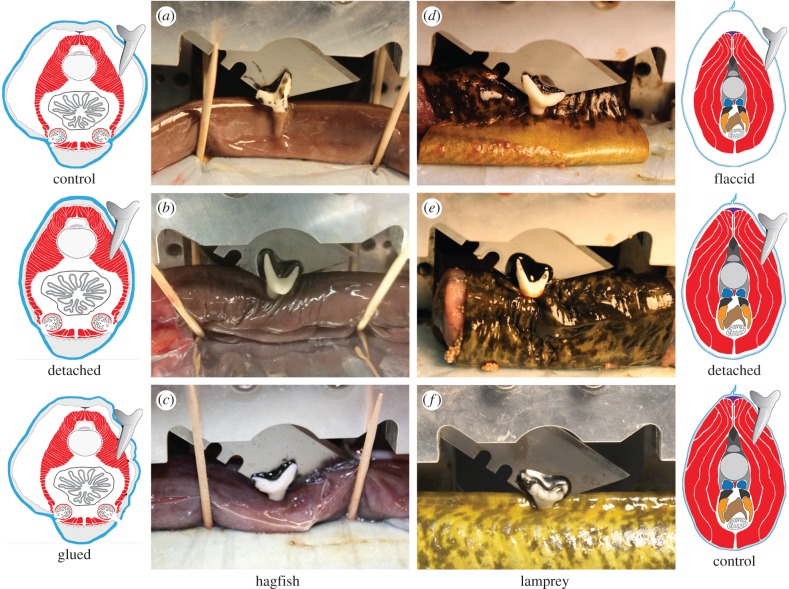
Figure 2.
Results of trials in which mako shark teeth were driven into hagfish and lamprey specimens with a spring-driven guillotine. (a) E. stoutii control specimens were punctured every time, but never sustained internal damage. (b) E. stoutii detached specimens were typically punctured through the skin and the underlying muscle. (c) E. stoutii-glued specimens similarly sustained damage to the skin and underlying muscle. (d) P. marinus specimens altered to have a flaccid skin akin to hagfishes were damaged less than unaltered controls. (e) Detached P. marinus specimens sustained damage to both their skin and underlying muscle, which was similar to the results for control P. marinus (f).
Table 1.
Results of shark tooth guillotine experiments. Top number in each box is the percentage of trials in which the tooth punctured the skin or underlying myotomal muscle. Middle number provides the total number of punctures divided by total number of trials, some of which were performed on different areas of the same animal. The bottom of each cell provides the total number of unique animals used for the trials within that treatment. Flaccid refers to hagfish in their natural state and lamprey with skin dissected away and moved to a smaller part of the body. Attached refers to lamprey in their natural state and hagfish in which the skin had been glued to the body. Detached, tight refers to hagfish in which a tube of skin was detached from the body and moved to a larger part of the body to reduce flaccidity, and lamprey in which a tube of skin was dissected away and then returned to the same position.
| Pacific hagfish E. stoutii | Atlantic hagfish M. glutinosa | sea lamprey P. marinus | ||||
|---|---|---|---|---|---|---|
| treatment | % skin punctured | % muscle punctured | % skin punctured | % muscle punctured | % skin punctured | % muscle punctured |
| flaccid | 100 (8/8) n = 6 |
0 (0/8) n = 6 |
100 (18/18) n = 6 |
0 (0/18) n = 6 |
41 (7/17) n = 7 |
24 (4/17) n = 7 |
| detached, tight | 97 (35/36) n = 6 |
86 (31/36) n = 6 |
97 (35/36) n = 6 |
92 (33/36) n = 6 |
100 (6/6) n = 4 |
100 (6/6) n = 6 |
| attached | 100 (13/13) n = 6 |
85 (11/13) n = 6 |
100 (13/13) n = 6 |
92 (12/13) n = 6 |
100 (13/13) n = 7 |
100 (13/13) n = 7 |
To rule out the possibility that the guillotine trials with mako shark teeth might not have been fast, forceful or sharp enough to cause damage, we replicated our experiments using freshly dead sea lamprey (Petromyzon marinus). P. marinus is an ideal control for this study, as lampreys are the closest living relatives of the hagfishes [13], they have an elongate body like hagfishes, and like most fishes, lamprey have a skin that is firmly attached to the underlying myotomal muscle (figure 2). In the natural state, P. marinus specimens were punctured through both the skin and underlying muscle in every trial. To test whether a lack of adhesion between the skin and musculature could reduce damage from a shark tooth, we dissected away a cylinder of skin and returned it to the same location; both the skin and muscle were punctured in every trial. To test whether flaccid skin could confer protection in P. marinus, we dissected away a cylinder of skin and moved it to a smaller area of the lamprey or an area where the gonads had been removed. In these flaccid lamprey specimens, the skin was punctured only 40% of the time and the underlying muscle was punctured only 24% of the time.
Our data do not support the hypothesis that hagfishes survive initial bites from predators by having exceptionally puncture-resistant skin. For E. stoutii skin, the force to puncture was unremarkable compared to other fish species, but the extension at puncture was higher, which is likely a result of the lack of stiff scales in hagfish skin. In the shark tooth guillotine experiments, hagfish skin was punctured in almost every trial, regardless of treatment (table 1). These results are consistent with observations by Zintzen et al. of a hagfish that was bitten by a kitefin shark (Dalatias licha) [2]. During this encounter, the skin was clearly punctured in one location where blood loss was evident, but there was no indication of any internal damage as the hagfish swam away seemingly without any difficulty.
Our results suggest that effective avoidance of internal damage from a penetrating tooth requires skin that is both unattached and flaccid. We propose that a sizable slack volume in hagfishes, combined with minimal attachment of the skin to the muscle, allows the body to slip out of harm's way, even when the skin is punctured. A strategy that involves possible puncture of skin associated with a capacious subcutaneous blood sinus would appear to put hagfishes at great risk of blood loss after an attack. However, hagfishes possess a low-pressure vascular system with multiple decentralized hearts [14]. The blood pressure in the venous SCS, especially considering its flaccidity, is likely to be barely above ambient, and therefore excessive blood loss from puncture wounds is unlikely, especially after clotting begins. In fact, minimizing blood loss from frequent puncture wounds may be one of the selective pressures that led to hagfishes to possess (or at least retain) a decentralized, low-pressure vascular system. If the hagfish lifestyle involves surviving attacks from sharp-toothed predators, examination of the skin of wild hagfishes for evidence of scarring may provide additional information about the frequency of such attacks.
Our shark tooth guillotine provides us with a simple measure of the effects of skin attachment and flaccidity on damage resistance in two species of hagfishes and one species of lamprey, but it does not provide a detailed description of the biomechanical interactions between hagfishes and shark predators. During a real-life interaction between a shark predator and a hagfish, the hagfish must contend with more than a single tooth, as well as teeth coming at it from two sides. Previous researchers have shown using razor blade physical models that multiple teeth can increase cutting efficiency by helping to focus cutting forces on a smaller volume of prey tissue [15]. Indeed, in preliminary trials using opposing v-shaped arrangements of razor blades with only a narrow lateral displacement, we were able to cleanly cut hagfishes in two. While interesting, these trials were too effective at cutting, and therefore not helpful for helping us discover the mechanisms by which hagfishes avoid damage from shark teeth. Future work on the effects of multiple tooth arrangements, as well as the effectiveness of different tooth morphologies from diverse species is sure to provide new insights into the fascinating predator–prey interactions among hagfishes and their fish predators. Moreover, a deeper understanding of the mechanisms elucidated here may inspire new strategies for the design of protective clothing or other products that can minimize damage from sharp objects.
Our results have implications for understanding not only the hagfish niche, but also how it may have evolved. Namely, our results suggest that the evolution of gill-clogging slime as a defence against biting fish predators is unlikely to have pre-dated the evolution of the hagfish's flaccid skin, as defensive slime would likely have little impact on fitness if the hagfish could not survive an initial attack. More specifically, our results suggest that, when it comes to defending themselves against biting sharks, slime release by hagfishes may function specifically to prevent the subsequent head shaking that many sharks employ after an initial bite to tear apart or subdue prey [16–20]. While the flaccid skin of hagfishes may be important in behaviours such as knot-tying [12] and burrowing [9], our results suggest it also plays an important role in predator defence.
2. Methods
Pacific hagfish (E. stoutii) were obtained from the Bamfield Marine Sciences Centre in Bamfield, British Columbia and housed as described in Winegard & Fudge [21]. Atlantic hagfish (M. glutinosa) were obtained, euthanized and used at the Shoals Marine laboratory in Maine. Freshly euthanized lamprey (P. marinus) were collected from the Humber River in Toronto, ON and euthanized shortly after capture. Skin samples of Pacific hagfish and 21 other fish species were tested for puncture resistance by being clamped between two plastic rings and punctured using a size 6 insect dissection pin driven by a model 3343 Instron Universal Testing machine (Illinois Tool Works Inc., Norwood, MA). All species tested possess scales, and none of the skin samples were de-scaled prior to testing. Species used for puncture tests were: (Hexagrammos stelleri (n = 3), Carcharhinus plumbeus (n = 1), Citharichthys sordidus (n = 1), Myoxocephalus polyacanthocephalus (n = 3), Ophiodon elongatus (n = 3), Embiotoca lateralis (n = 6), Apodichthys flavidus (n = 3), Aulorhynchus flavidus (n = 3), Blepsias cirrhosus (n = 3), Eopsetta jordani (n = 1), Gadus chalcogrammus (n = 1), Gasterosteus aculeatus (n = 3), Hexanchus griseus (n = 1), Isopsetta isolepis (n = 1), Lepidopsetta bilineata (n = 1), Liparis florae (n = 3), Lumpenus sagitta (n = 3), Lyopsetta exalis (n = 1), Psettichthys melanostictus (n = 1), Squalus suckleyi (n = 3), Oncorhynchus mykiss (n = 6). Puncture data (force, extension) were analysed with a general linear model in which species (hagfish, non-hagfish) was modelled as a fixed effect, and skin thickness as a covariate using SPSS v. 23. To test the flaccidity of the SCS, hagfish were euthanized using an overdose of buffered MS-222 (2 mg ml–1) in seawater for 30 min. The euthanized hagfish were weighed, measured (from their nostril to the tip of their tail) and their total body volume was determined using water displacement. To determine skin strain during inflation, six dots of India ink in two rows of three were applied to the skin caudal to the gill pouches at 0.5 cm increments using a sharp toothpick. Inflation experiments were then carried out to quantify the flaccidity of hagfish bodies by injecting hagfish Ringer's solution [22] (410 mM NaCl, 10 mM KCl, 14 mM MgSO4, 4 mM urea, 20 mM glucose, 10 mM HEPES, 4 mM CaCl2, pH 7.8) into the SCS in 5 ml increments using tubing continuous with a single-column manometer. For each 5 ml increment, the volume injected into the hagfish was calculated by subtracting out the additional volume that entered the manometer column. Slack volume was defined as the volume of fluid that can be injected before the SCS is ‘full', which was detected by monitoring the pressure as fluid was injected. The internal pressure of the hagfish was calculated using the density of the solution, the column height and the acceleration due to gravity (9.8066 m s−2). For every 10 ml of fluid injected, skin strain was calculated by measuring the distance between the six India ink dots using digital calipers. The experiment was terminated when fluid began seeping out of the skin, slime glands, cloaca or gill pouches. For guillotine experiments, the hagfish were euthanized by clove oil anaesthesia [21], followed by decapitation. The custom guillotine consisted of a metal mouton set on high precision rails, and driven by a spring that could be compressed and held in place by a pin. Pulling out the pin resulted in downward acceleration of the mouton, with the velocity at tooth contact (quantified from high speed videography) averaging approximately 0.57 m s−1. Mako shark (Isurus sp.) teeth were glued to the corner of metal plates using Clubmaker Shafting epoxy (Golfsmith International, Austin, TX), with the tooth attached to the plate so that the tip of the tooth was parallel to the direction of mouton travel. Each species of hagfish was tested in the guillotine in four states: (i) natural, (ii) glued, with the skin attached to the underlying musculature, (iii) detached, with the skin dissected from the body and then pinned to form a tight sleeve around the muscle and (iv) glued-control, with glue applied to the muscle and allowed to cure before the skin was replaced. For the glued treatment, a lateral flap of skin was created by cutting with scissors from the dorsal midline to the slime glands, caudally above the slime glands and back up to the dorsal midline. Small dots (approx. 300 µm) of Loctite 454 Prism (Henkel Corp., Rocky Hill, CT) were applied to every second myomere and on alternate myomeres above and below the lateral midline and the skin was replaced before the glue had a chance to cure, ensuring adhesion. The skin was held in place by silk thread sutures (size 3/0) along the bottom edge of the flap close to the slime glands. In the glue-control treatment, the same procedure was followed but the glue was allowed to cure before the skin was replaced, thereby minimizing adhesion between the skin and glue, but maintaining the presence of the hardened glue dots. Lamprey were tested in three states: (i) natural, (ii) detached, with its skin dissected away and replaced and (iii) flaccid, with the skin dissected away and moved to a smaller portion of the body. Hagfish and lamprey specimens were placed so that the tip of the tooth would make contact just lateral to the dorsal midline. Damage to the skin and parietal muscle was determined by dissection and visual inspection of the specimens after the trials.
Supplementary Material
Acknowledgements
We would like to thank Steve Wilson and Ian Moore from the University of Guelph Physics workshop for helping design and build the spring-driven guillotine. Thanks also to Matt Cornish and Mike Davies in the University of Guelph Aqualab for help with animal care, and Jessie Young from the Toronto and Region Conservation Authority for providing the lamprey used for this study. Thanks also to the staff at the Shoals Marine Laboratory and the Bamfield Marine Sciences Centre for help in acquiring hagfishes for this study.
Data accessibility
This article has no additional data.
Authors' contributions
S.B. and D.S.F. wrote the manuscript. S.B., J.L.S., A.P.S. and D.F. carried out the experiments and S.B., J.L.S. and D.F. analysed the data.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by NSERC Discovery and Accelerator grants to D.F.
References
- 1.Martini FH. 1998. The ecology of hagfishes. In The biology of hagfishes (eds Jorgensen JM, Lomholt JP, Weber RE, Malte H), pp. 57–77. London, UK: Chapman and Hall. [Google Scholar]
- 2.Zintzen V, Roberts CD, Anderson MJ, Stewart AL, Struthers CD, Harvey ES. 2011. Hagfish predatory behaviour and slime defence mechanism. Sci. Rep. 131, 1–6. ( 10.1038/srep00131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim J, Fudge DS, Levy N, Gosline JM. 2006. Hagfish slime ecomechanics: testing the gill-clogging hypothesis. J. Exp. Biol. 209, 702–710. ( 10.1242/jeb.02067) [DOI] [PubMed] [Google Scholar]
- 4.Böni L, Fischer P, Böcker L, Kuster S, Rühs PA. 2016. Hagfish slime and mucin flow properties and their implications for defense. Sci. Rep. 6, 30371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrew W, Hickman CP. 1974. Histology of the vertebrates (Illustrated). St Louis, MO: Mosby. [Google Scholar]
- 6.Vernerey FJ, Musiket K, Barthelat F. 2014. Mechanics of fish skin: a computational approach for bio-inspired flexible composites. Int. J. Solids Struct. 51, 274–283. ( 10.1016/j.ijsolstr.2013.10.001) [DOI] [Google Scholar]
- 7.Forster ME, Davison W, Satchell GH, Taylor HH. 1910. The subcutaneous sinus of the hagfish, Eptatretus cirrhatus and its relation to the central circulating blood volume. Comp. Biochem. Physiol. 93A, 607–612. [Google Scholar]
- 8.Parker TJ, Haswell WA. 1910. A text-book of zoology. London, UK: Macmillian. [Google Scholar]
- 9.Freedman CR, Fudge DS. 2017. Hagfish houdinis: biomechanics and behavior of squeezing through small openings. J. Exp. Biol. 220, 822–827. ( 10.1242/jeb.151233) [DOI] [PubMed] [Google Scholar]
- 10.Forster ME, Davie PS, Satchell GH, Wells RMG. 1988. Blood pressures and heart rates in swimming hagfish. Comp. Biochem. Physiol. A 89, 247–250. ( 10.1016/0300-9629(88)91087-0) [DOI] [Google Scholar]
- 11.Vogel S. 2013. Comparative biomechanics: life's physical world. Princeton, NJ: Princeton University Press. [Google Scholar]
- 12.Clark AJ, Crawford CH, King BD, Demas AM, Uyeno TA. 2016. Material properties of hagfish skin, with insights into knotting behaviors. Biol. Bull. 230, 243–256. ( 10.1086/BBLv230n3p243) [DOI] [PubMed] [Google Scholar]
- 13.Heimberg AM, Cowper-Sal R, Sémon M, Donoghue PCJ, Peterson KJ. 2010. MicroRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc. Natl Acad. Sci. USA 107, 19 379–19 383. ( 10.1073/pnas.1010350107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster ME, Axelsson M, Farell AP, Nilsson S. 1991. Cardiac function and circulation in hagfishes. Can. J. Zool. 69, 1985–1992. ( 10.1139/z91-277) [DOI] [Google Scholar]
- 15.Anderson PSL. 2009. The effects of trapping and blade angle of notched dentitions on fracture of biological tissues. J. Exp. Biol. 212, 3627–3632. ( 10.1242/jeb.033712) [DOI] [PubMed] [Google Scholar]
- 16.Springer S. 1961. Dynamics of the feeding mechanism of large galeoid sharks. Am. Zool. 1, 183–185. ( 10.1093/icb/1.2.183) [DOI] [Google Scholar]
- 17.Moss SA. 1977. Feeding mechanisms in sharks. Am. Zool. 17, 355–364. ( 10.1093/icb/17.2.355) [DOI] [Google Scholar]
- 18.Frazzetta TH, Prange CD. 1987. Movements of cephalic components during feeding in some requiem sharks (Carcharhiniformes: Carcharhinidae). Copeia 1987, 979–993. ( 10.2307/1445562) [DOI] [Google Scholar]
- 19.Motta P, Tricas T, Summers R. 1997. Feeding mechanism and functional morphology of the jaws of the lemon shark Negaprion brevirostris (Chondrichthyes, Carcharhinidae). J. Exp. Biol. 200, 2765–2780. [DOI] [PubMed] [Google Scholar]
- 20.Wilga C, Motta P. 1998. Conservation and variation in the feeding mechanism of the spiny dogfish Squalus acanthias. J. Exp. Biol. 201, 1345–1358. [DOI] [PubMed] [Google Scholar]
- 21.Winegard TM, Fudge DS. 2010. Deployment of hagfish slime thread skeins requires the transmission of mixing forces via mucin strands. J. Exp. Biol. 213, 1235–1240. ( 10.1242/jeb.038075) [DOI] [PubMed] [Google Scholar]
- 22.Gillis TE, Regan MD, Cox GK, Harter TS, Brauner CJ, Richards JG, Farrell AP. 2015. Characterizing the metabolic capacity of the anoxic hagfish heart. J. Exp. Biol. 218, 3754–3761. ( 10.1242/jeb.125070) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.