Abstract
Purpose
This study primarily aimed to investigate the short- and long-term remission rates of type 2 diabetes (T2D) in patients who underwent surgical treatment for gastric cancer, especially patients who were non-obese, and secondarily to determine the potential factors associated with remission.
Materials and Methods
We retrospectively reviewed the clinical records of patients with T2D who underwent radical gastrectomy for gastric cancer, from January 2008 to December 2012.
Results
T2D improved in 39 out of 70 (55.7%) patients at the postoperative 2-year follow-up and 21 of 42 (50.0%) at the 5-year follow-up. In the 2-year data analysis, preoperative body mass index (BMI) (P=0.043), glycated hemoglobin (A1C) level (P=0.039), number of anti-diabetic medications at baseline (P=0.040), reconstruction method (statistical difference was noted between Roux-en-Y reconstruction and Billroth I; P=0.035) were significantly related to the improvement in glycemic control. Unlike the results at 2 years, the 5-year data analysis revealed that only preoperative BMI (P=0.043) and A1C level (P=0.039) were statistically significant for the improvement in glycemic control; however, the reconstruction method was not.
Conclusions
All types of gastric cancer surgery can be effective in short- and long-term T2D control in non-obese patients. In addition, unless long-limb bypass is considered in gastric cancer surgery, the long-term glycemic control is not expected to be different between the reconstruction methods.
Keywords: Type 2 diabetes mellitus, Stomach neoplasms, Surgery, Reconstruction method, Glycemic index, Control
INTRODUCTION
The diagnosis of early gastric cancer (EGC) in Korea and Japan, which have the highest incidence rates, has increased by 1.7-fold [1], while the overall mortality rate for gastric cancer has been reduced by 21% because of the introduction of the National Cancer Screening Program [2]. Moreover, the 5-year survival rate for EGC has been reported to be >90% in recent decades. Therefore, improving the quality of life (QOL) after surgery and treating gastric cancer have been emphasized. To address this issue, a minimally invasive approach and function-preserving concept are the representative paradigms recently introduced in the field of gastric cancer treatment [3].
Along with this trend, the control of chronic disease can also be related to the QOL issue in patients with EGC. In particular, type 2 diabetes (T2D) is an important disease that should be carefully managed in long-term survivors, because of its association with blindness or chronic kidney disease and increased risk of coronary heart disease and stroke. In other words, even though EGC usually has good prognosis after surgical treatment, patients with T2D may undergo lifelong complications, including diabetic retinopathy, diabetic nephropathy, and other cardiovascular diseases [4,5,6,7,8]. However, since gastric cancer surgery has some similar fundamental components with bariatric surgery, its effect on T2D is promising.
According to several systematic reviews, bariatric surgery in patients with high body mass index (BMI) can lead to complete remission of diabetes in >90% of patients, along with reduced risk factors for cardiovascular diseases, such as hypertension and lipid abnormalities [9,10]. In addition, QOL typically improved during the first years of follow-up and seemed to stabilize 5 years after the operation [11,12]. Thus, bariatric surgery is considered as treatment for obese diabetic patients who do not respond to diet, drug therapy, and lifestyle modification. Furthermore, even for non-obese T2D patients (BMI of <35 kg/m2), surgical treatment has been reported to possibly facilitate glycemic control by reducing the need for insulin [13]. A systematic review showed a diabetes remission rate of 85% after bariatric surgery in non-obese T2D patients (BMI of <35 kg/m2) [14]. The effects of gastric cancer surgery are similar to that of bariatric surgery, as the gastrectomized and duodenum- or jejunum-bypassing structures are formed by a gastrectomy with gastrointestinal reconstruction. Therefore, considering the effects of bariatric surgery, diabetic remission may also be induced by gastric cancer surgery. Recently, this concept has been established as “onco-metabolic surgery,” because of the long-limb Roux-en-Y reconstruction in gastric cancer patients with T2D [15,16]. The main purpose of onco-metabolic surgery is to induce glycemic control by mimicking the structure of Roux-en-Y gastric bypass (RYGB), a widely accepted bariatric procedure.
However, few studies reported the long-term T2D improvement after gastric cancer surgery. This study primarily aimed to investigate the short- and long-term remission rates of T2D in patients who underwent surgical treatment for gastric cancer, especially non-obese patients, and secondarily to determine the potential factors associated with remission.
MATERIALS AND METHODS
Between January 2008 and December 2012, the medical records of patients with T2D who underwent radical gastrectomy for gastric adenocarcinoma were retrospectively reviewed. We collected records on clinicopathologic characteristics and treatment outcomes in these patients and examined data including age, sex, preoperative BMI, duration of T2D, preoperative glycated hemoglobin (A1C) level, diabetes medication use, reconstruction method, and pathologic stage (according to the 7th edition of the American Joint Committee on Cancer [AJCC] staging system) [17].
Patients with the following criteria were excluded: 1) BMI of ≥30 kg/m2, 2) previous abdominal surgery, 3) pathologic stage IV based on the 7th edition of the AJCC staging system [17], 4) any complications associated with gastrectomy, 5) other malignancies diagnosed during the follow-up period, and 6) death before the end of the 2-year follow-up period.
Division of patients
Patients were divided into 2 groups according to glycemic control status at 2 and 5 years after gastrectomy. Patients with improved T2D control were included in the improved control (IC) group, and those with no improvement were included in the not improved control (NC) group.
The IC group consisted of patients who experienced complete remission, partial remission, and improved T2D based on A1C and fasting blood glucose (FBG) levels. Complete remission was defined as normal measurements for glucose metabolism (A1C of <6%, FBG of <100 mg/dL) without diabetic medications for at least 1 year. Partial remission was defined as sub-diabetic hyperglycemia (A1C of 6%–6.4%, FBG of 100–125 mg/dL) without diabetic medications for at least 1 year. Improved T2D was defined as reduced A1C (by >1%) and FBG (by >25 mg/dL) levels or diabetic medication dosages. The NC group consisted of patients who experienced relapse or no change in the glycemic status. Relapse was defined as FBG or A1C level in the diabetic range (FBG of ≥126 mg/dL and A1C of ≥6.5%) or the need for anti-diabetic medication after initial, complete, or partial remission.
Data analysis
Patient characteristics such as age, sex, BMI, incidence of hypertension, duration of T2D, A1C level, FBG level, medication status, reconstruction method, and cancer stage were compared between the IC and NC groups.
To investigate the significant factors related to improvement in glycemic control, univariate and multivariate analyses were performed for 2- and 5-year follow-up data. Initially, factors known to be related to T2D, including age, sex, preoperative BMI, hypertension, preoperative medication, reconstruction method, gastrectomy range, and cancer stage were included in univariate analysis. Multivariate analysis included only the factors that showed statistical significance in univariate analysis.
Meanwhile, as a supplementary analysis, we divided the patients according to the reconstruction method (Billroth I, Billroth II, or Roux-en-Y reconstruction) and analyzed the rate of T2D improvement.
For all patients analyzed in this study, glycemic status assessment was adapted from the American Diabetes Association [18,19] and the American Society for Metabolic and Bariatric Surgery recommendations [20].
Surgical procedures for all study patients
Lymphadenectomy for curative distal gastrectomy was performed based on the 2010 Japanese Gastric Cancer Treatment Guidelines (version 3) [21]. After completion of lymphadenectomy, the range of gastrectomy was determined based on the size and location of the tumor. Billroth I, Billroth II, or total gastrectomy with Roux-en-Y reconstruction (RYTG) was performed to recover the gastrointestinal continuity [22].
In patients who underwent Billroth I reconstruction, the duodenum was mobilized using Kocher maneuver, and tension-free anastomosis was performed. In Billroth II reconstruction, anastomosis was performed using a jejunal loop at approximately 15–20 cm distal to the ligament of Treitz. For RYTG, the jejunum was transected approximately 20 cm distal to the ligament of Treitz, and jejunojejunostomy was performed approximately 40 cm distal to the esophagojejunal anastomosis. Thus, the length of the totally bypassed limb is approximately 60 cm in RYTG.
Statistical analysis
The SPSS software version 24.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses. All continuous data are presented as the mean±standard deviation and were analyzed using the independent sample t-test. Categorical data are presented as percentages and analyzed using the χ2 test. Binary logistic regression models were used for univariate and multivariate analyses. For all data analyses, P-value of <0.05 was considered statistically significant.
Ethical review
Approval to perform research on human subjects in this study was provided by the Institutional Review Board of Korea University Anam Hospital (registration number: ED14229).
RESULTS
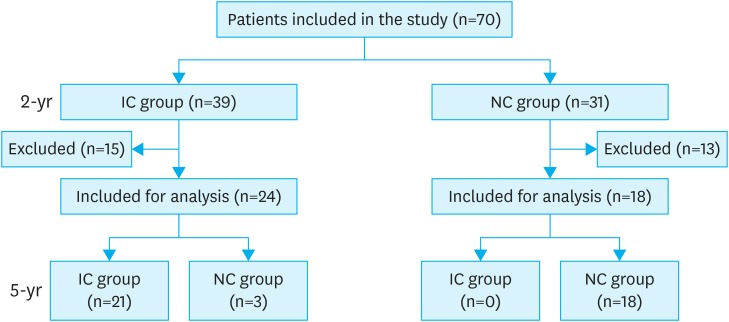
A total of 70 patients were included in the 2-year data analysis. Twenty-eight patients were excluded from the 5-year follow-up data: 20 patients were lost to follow-up, 2 had hepatic metastasis, and 2 had pulmonary metastasis, 3 died during the observation period, and 1 developed pancreatic cancer. Finally, 42 patients were included in the 5-year data analysis with the median follow-up time of 64 months (Fig. 1).
Fig. 1.
Flow diagram of patients in the study.
IC = improved control; NC = not improved control.
Table 1 shows the baseline characteristics of 70 initially included patients. Among them, 19 patients (27.1%) underwent subtotal gastrectomy with Billroth I, 33 (47.1%) with Billroth II, and 18 (25.7%) with RYTG. Patients consisted of 48 (68.6%) men and 22 (31.4%) women with a mean age of 68.7±10.3 years, mean baseline BMI of 25.1±3.1 kg/m2, median diabetes duration of 12.7±6.1 years, and mean baseline A1C of 6.9%±1.1%.
Table 1. Demographic and clinicopathologic data (n=70).
| Variables | Value | |
|---|---|---|
| Age (yr) | 68.7±10.3 (45–88) | |
| Sex (%) | ||
| Male | 48 (68.6) | |
| Female | 22 (31.4) | |
| BMI (kg/m2) | 25.1±3.1 (15.8–29.5) | |
| Hypertension (%) | 29 (41.4) | |
| Diabetes factors | ||
| Duration (yr) | 12.7±6.1 (1–36) | |
| A1C (%) | 6.9±1.1 (5.4–12.1) | |
| Fasting glucose (mg/dL) | 139.6±40.0 (72–245) | |
| Preoperative medication (%) | ||
| Single | 31 (44.3) | |
| Multiple | 39 (55.7) | |
| Insulin injection | 5 (7.1) | |
| Reconstruction method (%) | ||
| Billroth I | 19 (27.1) | |
| Billroth II | 33 (47.1) | |
| RYTG | 18 (25.7) | |
| Cancer stage (%) | ||
| I | 47 (67.1) | |
| II | 10 (14.3) | |
| III | 13 (18.6) | |
Values are presented as number (%) or mean±standard deviation unless otherwise indicated.
BMI = body mass index; A1C = glycated hemoglobin; RYTG = total gastrectomy with Roux-en-Y reconstruction.
Comparison of characteristics between the 2 groups
Table 2 compares the characteristics of IC and NC groups. In the 2-year data analysis, the preoperative BMI showed no significant difference between the IC and NC groups (25.3±3.6 and 23.8±3.2 kg/m2, respectively; P=0.073). The A1C level (6.6%±0.8% and 7.5%±1.4%, respectively; P=0.002) and the use of multiple anti-diabetic medications (42.9% and 77.4%, respectively; P=0.004) were significantly lower in the IC than that in the NC group. In the 5-year data analysis, the preoperative BMI was significantly higher in the IC than that in the NC group (26.4±3.0 and 24.1±2.9 kg/m2, respectively; P=0.016), and the A1C level was significantly lower in the IC that in the NC group (6.4%±0.9% and 7.2%±1.1%, respectively; P=0.022).
Table 2. Comparison of demographic and clinicopathologic data between the IC and NC group.
| Variables | 2-yr (n=70) | 5-yr (n=42) | |||||
|---|---|---|---|---|---|---|---|
| IC group (n=39) | NC group (n=31) | P | IC group (n=21) | NC group (n=21) | P | ||
| Age (yr) | 70.5±8.9 | 68.7±12.0 | 0.488 | 71.4±8.2 | 67.0±11.9 | 0.173 | |
| Sex (male:female) | 2.3:1 | 2.1:1 | 0.894 | 2.5:1 | 4.25:1 | 0.469 | |
| BMI (kg/m2) | 25.3±3.6 | 23.8±3.2 | 0.073 | 26.4±3.0 | 24.1±2.8 | 0.016 | |
| Incidence of hypertension (%) | 24 (61.5) | 19 (61.3) | 0.983 | 15 (71.4) | 14 (66.7) | 0.739 | |
| Diabetes factors | |||||||
| Duration (yr) | 15.1±8.5 | 15.4±8.4 | 0.918 | 13.9±7.3 | 12.1±6.2 | 0.389 | |
| A1C (%) | 6.6±0.8 | 7.5±1.4 | 0.002 | 6.4±0.9 | 7.2±1.1 | 0.022 | |
| Fasting glucose (mg/dL) | 136.6±37.4 | 143.1±43.6 | 0.624 | 136.4±36.9 | 142.3±43.2 | 0.649 | |
| Preoperative medication (%) | 0.004 | 0.061 | |||||
| Single medication | 20 (57.1) | 7 (22.6) | 12 (57.1) | 6 (28.6) | |||
| Multiple medications | 15 (42.9) | 24 (77.4) | 9 (42.9) | 15 (71.4) | |||
| Insulin injection | 2 (5.1) | 3 (9.7) | 0.463 | 2 (9.5) | 3 (14.3) | 0.634 | |
| Reconstruction method (%) | 0.092 | 0.904 | |||||
| Billroth I | 7 (17.9) | 12 (38.7) | 4 (19.0) | 5 (23.8) | |||
| Billroth II | 19 (48.7) | 14 (45.2) | 11 (52.4) | 11 (52.4) | |||
| RYTG | 13 (33.3) | 5 (16.1) | 6 (28.6) | 5 (23.8) | |||
| Cancer stage (%) | 0.953 | 0.725 | |||||
| I | 26 (66.7) | 21 (67.7) | 13 (61.9) | 15 (71.4) | |||
| II | 6 (15.4) | 4 (12.9) | 5 (23.8) | 3 (14.3) | |||
| III | 7 (17.9) | 6 (19.4) | 3 (14.3) | 3 (14.3) | |||
Values are presented as number (%) or mean±standard deviation unless otherwise indicated. IC group consisted of patients who underwent complete remission, partial remission, and improved T2D mellitus based on A1C and FBG levels. NC group consisted of patients who experienced relapse or no change in the glycemic status.
IC = improved control; NC = not improved control; BMI = body mass index; A1C = glycated hemoglobin; RYTG = total gastrectomy with Roux-en-Y reconstruction; T2D = type 2 diabetes; FBG = fasting blood glucose.
Short- and long-term predictors for diabetes remission
A total of 39 of the 70 (55.7%) patients had improved diabetes during the postoperative 2-year follow-up, and 21 of 42 (50%) during the 5-year follow-up (Table 2).
In the 2-year follow-up, multivariate analysis revealed that preoperative BMI (odds ratio [OR], 1.27; 95% confidence interval [CI], 1.01–1.61; P=0.043), preoperative A1C level (OR, 0.45; 95% CI, 0.21–0.96; P=0.039), and number of diabetic medications (OR, 0.22; 95% CI, 0.05–0.93; P=0.040) were significantly related to T2D improvement (Table 3). Regarding the reconstruction method, RYTG was more effective in improving T2D than Billroth I reconstruction (OR, 8.93; 95% CI, 1.17–68.36; P=0.035), whereas the difference in T2D improvement between Billroth I and Billroth II was statistically insignificant.
Table 3. Predictors of diabetes improvement at postoperative 2-year and 5-year follow-up.
| Variables | 2-yr (n=70) | 5-yr (n=42) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Age (yr) | 1.02 (0.97–1.07) | 0.482 | 1.05 (0.98–1.11) | 0.174 | |||||
| Sex, female (vs. male) | 0.99 (0.34–2.57) | 0.894 | 1.70 (0.40–7.20) | 0.471 | |||||
| Preoperative BMI | 1.14 (0.98–1.14) | 0.082 | 1.27 (1.01–1.61) | 0.043 | 1.32 (1.04–1.68) | 0.024 | 1.38 (1.05–1.81) | 0.021 | |
| Hypertension | 1.01 (0.38–2.66) | 0.983 | 1.25 (0.34–4.64) | 0.739 | |||||
| Diabetes factors | |||||||||
| Duration (yr) | 0.99 (0.94–1.06) | 0.916 | 1.04 (0.95–1.15) | 0.382 | |||||
| A1C (%) | 0.41 (0.22–0.76) | 0.004 | 0.45 (0.21–0.96) | 0.039 | 0.47 (0.23–0.93) | 0.031 | 0.41 (0.17–0.99) | 0.047 | |
| Fasting glucose (mg/dL) | 0.99 (0.98–1.01) | 0.610 | 0.99 (0.98–1.01) | 0.643 | |||||
| Preoperative medication (%) | 0.22 (0.08–0.64) | 0.006 | 0.22 (0.05–0.93) | 0.040 | 0.30 (0.08–1.08) | 0.066 | 0.57 (0.13–2.61) | 0.470 | |
| Insulin, yes (vs. no) | 0.51 (0.08–3.23) | 0.470 | 0.63 (0.09–4.23) | 0.636 | |||||
| Reconstruction method (%) | |||||||||
| Billroth I | Reference | Reference | |||||||
| Billroth II | 2.33 (0.73–7.42) | 0.154 | 3.67 (0.65–20.59) | 0.140 | 1.25 (0.26–5.94) | 0.779 | 2.98 (0.35–25.27) | 0.317 | |
| RYTG | 4.46 (1.11–17.90) | 0.035 | 8.93 (1.17–68.36) | 0.035 | 1.50 (0.26–8.82) | 0.654 | 2.46 (0.26–23.60) | 0.436 | |
| Gastrectomy range (total vs. subtotal) | 2.60 (0.81–8.34) | 0.108 | 0.78 (0.20–3.11) | 0.726 | |||||
| Weight change | 1.14 (0.98–1.33) | 0.089 | 1.02 (0.91–1.13) | 0.774 | |||||
| Cancer stage (%) | |||||||||
| I | Reference | Reference | |||||||
| II | 1.21 (0.30–4.86) | 0.787 | 1.92 (0.38–9.65) | 0.427 | |||||
| III | 0.94 (0.28–3.23) | 0.925 | 1.15 (0.20–6.74) | 0.874 | |||||
OR = odds ratio; CI = confidence interval; BMI = body mass index; A1C = glycated hemoglobin; RYTG = total gastrectomy with Roux-en-Y reconstruction.
A total of 42 patients were included in the 5-year follow-up (Fig. 1). In multivariate analysis, the preoperative BMI (OR, 1.38; 95% CI, 1.05–1.81; P=0.021) and A1C level (OR, 0.41; 95% CI, 0.17–0.99; P=0.047) were significantly related to T2D improvement (Table 3). However, regarding the reconstruction method, RYTG and Billroth I showed no significant difference in T2D improvement (OR, 2.46; 95% CI, 0.26–23.60; P=0.436). In addition, the number of diabetic medications (OR, 2.60; 95% CI, 0.81–8.34; P=0.108) was insignificantly related to T2D improvement.
Glycemic control
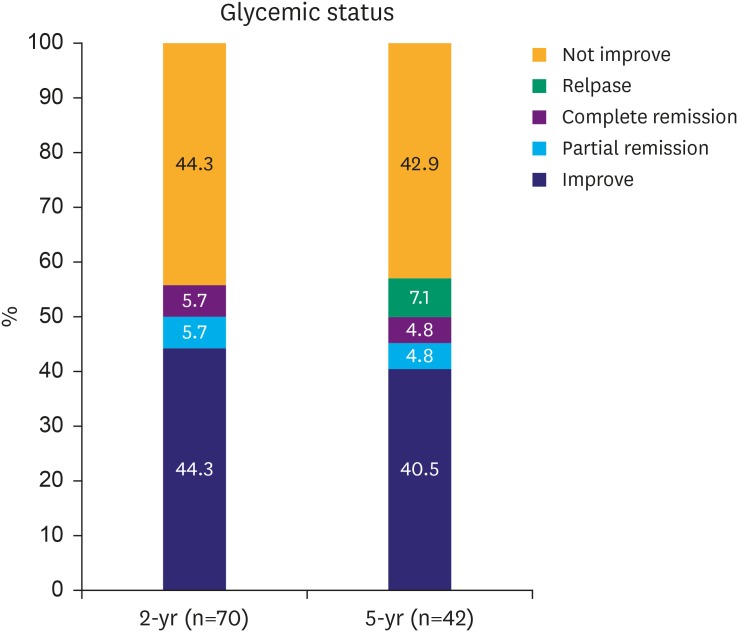
Overall, 39 of 70 (55.7%) and 21 of 42 (50.0%) patients were included in the IC group at the 2- and 5-year follow-up, respectively. In the 5-year outcome, 40.5% of patients had improved T2D, 4.8% had partial remission, and 4.8% had complete remission. Three patients (7.1%) who experienced improved T2D had a relapse during the long-term follow-up (Fig. 2).
Fig. 2.
Short- and long-term diabetes remission and relapse rate.
Short- and long-term follow-up data showed improved glycemic control in 55.7% and 50.1% of patients. In the long-term, 50% of patients underwent glycemic control that was unchanged or worse compared with the baseline.
A subgroup analysis was conducted between the group with persistent T2D improvement and with relapse; however, differences in the variables between the 2 groups were statistically insignificant.
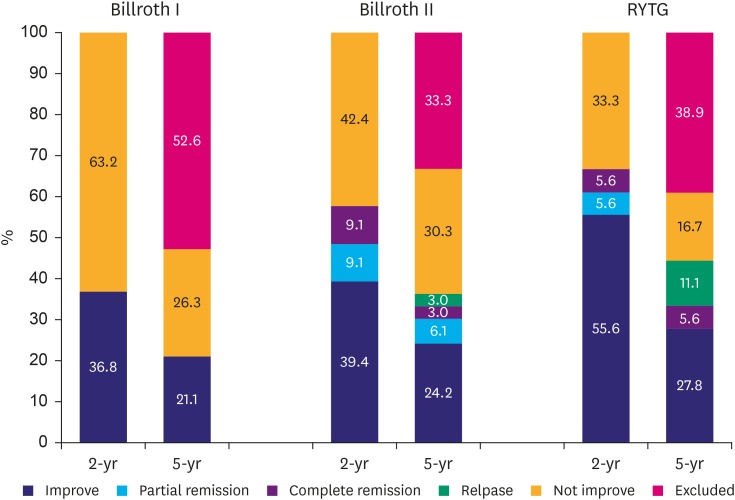
Supplementary analysis comparing outcomes between Billroth I, Billroth II, and RYTG (Fig. 3)
Fig. 3.
Impact of operation methods on diabetes improvement at postoperative 2 and 5 years.
During the 2-year follow-up, patients who underwent RYTG had significantly higher diabetes improvement rate compared with those who underwent Billroth I (P=0.035).However, the difference in diabetes improvement rate between RYTG and Billroth Iat 5-year follow-up was insignificant.
RYTG =total gastrectomy with Roux-en-Y reconstruction.
A total of 7 out of 19 patients who underwent Billroth I showed improvement at the 2-year follow-up, and all 4 patients, except for the 3 who were excluded from the 5-year analysis (Fig. 1), maintained the improved status during the 5-year follow-up.
A total of 13 out of 33 Billroth II patients experienced improved T2D, 3 had partial remission, and 3 had complete remission for 2 years. One patient who had complete remission showed a relapse during the 5-year follow-up.
In patients who underwent RYTG, 11 showed improved T2D at the 2-year follow-up, including one with partial remission and one with complete remission. In addition, relapsed patients at the 5-year follow-up included one of the improved patients and one with partial remission at the 2-year outcome.
DISCUSSION
Bariatric surgery is designed for weight loss in the morbidly obese population and is also known to have a metabolic effect that can improve diabetes [23,24]. In addition, one meta-analysis found improvement in 86.6% of T2D patients who received bariatric surgery [25]. The biliopancreatic diversion or duodenal switch, one of the methods of bariatric surgery, resulted in diabetic remission in 93.0%–97.9% of patients [9].
These promising results of bariatric surgery are expected to be similar to that of gastric cancer surgery. Bariatric procedures have a remarkably similar structure with that of Billroth II and Roux-en-Y reconstruction methods performed during gastric cancer surgery.
In Korea, the prevalence rate of gastric cancer is higher than that in Western countries [26,27], but obesity (BMI of >30 kg/m2) is uncommon. However, few studies examined the long-term effects of gastric cancer surgery on diabetes improvement in non-obese patients. Therefore, we studied the glycemic control in diabetic patients who underwent gastric cancer surgery for 2 and 5 years postoperatively.
In our analysis, the preoperative A1C level, preoperative BMI, number of diabetic medications, and reconstruction method (RYTG vs. Billroth I) were positive predictors of diabetic control in the short-term follow-up. In particular, the preoperative A1C level was found to have a significant influence on diabetes improvement during the short- and long-term follow-up. This result is consistent with findings from previous studies [24,28,29]. Several reports showed that low A1C levels have a positive effect on diabetes improvement after bariatric surgery [24,29]. Moreover, preoperative A1C levels may be related to the severity of T2D in preoperative patients, suggesting that serious progression of diabetes tends to lower the effectiveness of bariatric surgery. In other words, early surgical intervention may have a positive effect on the improvement of diabetes.
Moreover, some previous outcomes are also consistent with our results, which suggested that a preoperative high BMI was associated with improvement of diabetes in both short- and long-term follow-up [30]. High BMI has been associated with increased residual β-cell function, and a correlation between obesity and β-cell mass has been found in some studies [31,32].
However, the reconstruction method (Billroth I, Billroth II, or RYTG) did not affect T2D improvement in the long-term follow-up, whereas the 2-year follow-up data analysis revealed a significant relationship between the reconstruction method and T2D control after gastric cancer surgery. This result implies an important difference between gastric cancer surgery and bariatric surgery in terms of T2D control.
Regarding the short-term glycemic control, gastric cancer surgery followed the clinical characteristics of sleeve gastrectomy or RYGB. Although several studies have suggested metabolic effects of sleeve gastrectomy [28,33,34], T2D improvement after sleeve gastrectomy is mainly associated with weight loss. Therefore, in non-obese T2D patients, sleeve gastrectomy and RYGB are not expected to have similar results in terms of glycemic control [35]. Sleeve gastrectomy only has the restrictive effect of reducing caloric intake, whereas RYGB has a bypass effect, which can be explained by the hindgut and foregut theories on the mechanism of diabetes improvement after bariatric surgery. The foregut theory suggests that duodenojejunal bypass prevents the release of signals that promote insulin resistance. Duodenojejunal bypass surgery was tested in non-obese Goto-Kakizaki rats, which showed diabetes improvement after surgery [36]. In addition, the hindgut theory states that fast migration of nutrients to the distal small bowel through the reconstruction increases the secretion of hormones such as glucagon-like peptide-1, a critical factor in weight loss and diabetes remission [23,37]. Among the reconstruction methods for gastric cancer surgery, RYTG might induce similar hormonal responses because of its similarity with the bypassing structure of RYGB. On the other hand, Billroth I resembles sleeve gastrectomy in which the pancreas and duodenum are not bypassed; therefore, this method has a weaker metabolic effect on T2D in non-obese patients. In addition, although Billroth II does not completely bypass the pancreas and duodenum, this procedure sends lesser nutrients to the biliopancreatic limb than does Billroth I. Therefore, we hypothesized that RYTG and Billroth II are more effective in T2D control than Billroth I.
Furthermore, RYTG have been more effective on improving diabetes than Billroth I in the short-term follow-up. As T2D improvement did not differ between total and subtotal gastrectomy (Table 3), we concluded that gastrectomy range does not affect T2D improvement. In other words, RYTG produced better T2D control than Billroth I not because of the difference in remnant gastric volume but because of the duodenojejunal bypassing structure.
Nevertheless, in the 5-year outcome of this study, various reconstruction methods did not show any significant differences in terms of glycemic control. In other words, duodenojejunal bypassing structure of RYTG might have little effect on T2D control in the long-term follow-up.
Based on this result, the absence of “long-limb reconstruction” is considered to diminish the long-term effect of duodenojejunal bypassing structure. In RYGB, the length of the bypassed limb has been considered as an important key for T2D management [38,39], although studies reporting the relationship between the limb length and glycemic control have been limited. Thus, “long-limb reconstruction” was not used in this study, even though the length of the biliopancreatic limb in RYGB and Roux limb are approximately 200 and 150 cm, respectively [29,40,41]. Even though Kim et al. [40]. also showed T2D improvement after gastric cancer surgery, they only used long-limb Roux-en-Y reconstruction. Onco-metabolic surgery, a concept recently introduced for gastric cancer patients with T2D, embeds long-limb Roux-en-Y reconstruction and therefore is expected to be more advantageous for long-term glycemic control than conventional gastric cancer surgery.
Although we previously suggested the significance of a surgical duodenal switch for the treatment of non-obese T2D patients [42], the long-term outcome of gastric cancer surgery on diabetes remission was not identified. However, this study evaluated the 2- and 5-year treatment outcomes on T2D patients who underwent gastric cancer surgery, which revealed a diminished long-term effect of duodenal bypassing structure. Based on our literature review, this is the first study to evaluate the long-term effects of gastric cancer surgery on T2D.
Meanwhile, this study reveals some limitations. First, preoperative medications and disability were not clearly addressed due to incomplete records used in the retrospective design. In addition, the number of patients in the study, particularly at 5 years, was too small for a significant multivariate regression analysis. These drawbacks should be addressed in future studies.
In conclusion, although the preoperative BMI, A1C level, number of diabetes medications used at baseline, and reconstruction method were significant predictors for T2D improvement in the short-term glycemic control, the reconstruction method may not have a significant effect on the long-term glycemic control. The discrepancy between short- and long-term outcomes may be due to the length of limbs in gastric cancer surgery. In other words, the hormonal effect of the bypassing structure may be attenuated over time, unless long-limb reconstruction is applied in gastric cancer surgery. Further long-term and large-scale studies are necessary to resolve this issue.
Footnotes
- Conceptualization: P.S.
- Data curation: L.T.H.
- Formal analysis: J.D.H.
- Investigation: L.T.H.
- Methodology: P.S.
- Project administration: L.C.M.
- Resources: P.S.
- Software: K.J.H.
- Supervision: M.Y.J.
- Validation: P.S.H.
- Visualization: J.Y.J.
- Writing - original draft: L.T.H.
- Writing - review & editing: L.C.M.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Choi KS, Jun JK, Suh M, Park B, Noh DK, Song SH, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the National Cancer Screening Programme in Korea. Br J Cancer. 2015;112:608–612. doi: 10.1038/bjc.2014.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology. 2017;152:1319–1328.e7. doi: 10.1053/j.gastro.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Saito T, Kurokawa Y, Takiguchi S, Mori M, Doki Y. Current status of function-preserving surgery for gastric cancer. World J Gastroenterol. 2014;20:17297–17304. doi: 10.3748/wjg.v20.i46.17297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tun NN, Arunagirinathan G, Munshi SK, Pappachan JM. Diabetes mellitus and stroke: a clinical update. World J Diabetes. 2017;8:235–248. doi: 10.4239/wjd.v8.i6.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Retinopathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S84–S87. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- 6.Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 7.Ritz E, Stefanski A. Diabetic nephropathy in type II diabetes. Am J Kidney Dis. 1996;27:167–194. doi: 10.1016/s0272-6386(96)90538-7. [DOI] [PubMed] [Google Scholar]
- 8.Wolf G, Ritz E. Diabetic nephropathy in type 2 diabetes prevention and patient management. J Am Soc Nephrol. 2003;14:1396–1405. doi: 10.1097/01.asn.0000065639.19190.cf. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 10.Cho JM, Kim HJ, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. Effect of sleeve gastrectomy on type 2 diabetes as an alternative treatment modality to Roux-en-Y gastric bypass: systemic review and meta-analysis. Surg Obes Relat Dis. 2015;11:1273–1280. doi: 10.1016/j.soard.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Andersen JR, Aasprang A, Karlsen TI, Natvig GK, Våge V, Kolotkin RL. Health-related quality of life after bariatric surgery: a systematic review of prospective long-term studies. Surg Obes Relat Dis. 2015;11:466–473. doi: 10.1016/j.soard.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Risstad H, Søvik TT, Engström M, Aasheim ET, Fagerland MW, Olsén MF, et al. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: a randomized clinical trial. JAMA Surg. 2015;150:352–361. doi: 10.1001/jamasurg.2014.3579. [DOI] [PubMed] [Google Scholar]
- 13.Baskota A, Li S, Dhakal N, Liu G, Tian H. Bariatric surgery for type 2 diabetes mellitus in patients with BMI <30 kg/m2: a systematic review and meta-analysis. PLoS One. 2015;10:e0132335. doi: 10.1371/journal.pone.0132335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried M, Ribaric G, Buchwald JN, Svacina S, Dolezalova K, Scopinaro N. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI <35 kg/m2: an integrative review of early studies. Obes Surg. 2010;20:776–790. doi: 10.1007/s11695-010-0113-3. [DOI] [PubMed] [Google Scholar]
- 15.ClinicalTrials.gov. (US) Nutritional safety and metabolic benefits of oncometabolic surgery for obese gastric cancer patients (ONCOMETAB) [Internet] Bethesda (MD): National Institutes of Health; 2017. [cited 2017 Sep 26]. Available from: https://clinicaltrials.gov/ct2/show/NCT03067012. [Google Scholar]
- 16.Lee CM, Kim JH. Surgical treatment of morbid obesity. Korean J Helicobacter Up Gastrointest Res. 2017;17:72–78. [Google Scholar]
- 17.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes. 2016;34:3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Standards of medical care in diabetes-2017 abridged for primary care providers. Clin Diabetes. 2017;35:5–26. doi: 10.2337/cd16-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis. 2015;11:489–506. doi: 10.1016/j.soard.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 22.Lee CM, Park S, Park SH, Jang YJ, Kim SJ, Mok YJ, et al. A comparison between two methods for tumor localization during totally laparoscopic distal gastrectomy in patients with gastric cancer. Ann Surg Treat Res. 2016;91:112–117. doi: 10.4174/astr.2016.91.3.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho YM. A gut feeling to cure diabetes: potential mechanisms of diabetes remission after bariatric surgery. Diabetes Metab J. 2014;38:406–415. doi: 10.4093/dmj.2014.38.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pak J, Kwon Y, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. Impact of gastrointestinal bypass on nonmorbidly obese type 2 diabetes mellitus patients after gastrectomy. Surg Obes Relat Dis. 2015;11:1266–1272. doi: 10.1016/j.soard.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 28.Aminian A, Brethauer SA, Andalib A, Punchai S, Mackey J, Rodriguez J, et al. Can sleeve gastrectomy “cure” diabetes? Long-term metabolic effects of sleeve gastrectomy in patients with type 2 diabetes. Ann Surg. 2016;264:674–681. doi: 10.1097/SLA.0000000000001857. [DOI] [PubMed] [Google Scholar]
- 29.Kim JW, Kim KY, Lee SC, Yang DH, Kim BC. The effect of long Roux-en-Y gastrojejunostomy in gastric cancer patients with type 2 diabetes and body mass index < 35 kg/m(2): preliminary results. Ann Surg Treat Res. 2015;88:215–221. doi: 10.4174/astr.2015.88.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon Y, Jung Kim H, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. A systematic review and meta-analysis of the effect of Billroth reconstruction on type 2 diabetes: a new perspective on old surgical methods. Surg Obes Relat Dis. 2015;11:1386–1395. doi: 10.1016/j.soard.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 32.Retnakaran R, Zinman B. Short-term intensified insulin treatment in type 2 diabetes: long-term effects on β-cell function. Diabetes Obes Metab. 2012;14(Suppl 3):161–166. doi: 10.1111/j.1463-1326.2012.01658.x. [DOI] [PubMed] [Google Scholar]
- 33.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao RS, Kini S. Diabetic and bariatric surgery: a review of the recent trends. Surg Endosc. 2012;26:893–903. doi: 10.1007/s00464-011-1976-7. [DOI] [PubMed] [Google Scholar]
- 36.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas S, Schauer P. Bariatric surgery and the gut hormone response. Nutr Clin Pract. 2010;25:175–182. doi: 10.1177/0884533610361739. [DOI] [PubMed] [Google Scholar]
- 38.Kao YH, Lo CH, Huang CK. Relationship of bypassed limb length and remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2012;8:e82–e84. doi: 10.1016/j.soard.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Pinheiro JS, Schiavon CA, Pereira PB, Correa JL, Noujaim P, Cohen R. Long-long limb Roux-en-Y gastric bypass is more efficacious in treatment of type 2 diabetes and lipid disorders in super-obese patients. Surg Obes Relat Dis. 2008;4:521–525. doi: 10.1016/j.soard.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Kim WS, Kim JW, Ahn CW, Choi SH. Resolution of type 2 diabetes after gastrectomy for gastric cancer with long limb Roux-en Y reconstruction: a prospective pilot study. J Korean Surg Soc. 2013;84:88–93. doi: 10.4174/jkss.2013.84.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boza C, Muñoz R, Salinas J, Gamboa C, Klaassen J, Escalona A, et al. Safety and efficacy of Roux-en-Y gastric bypass to treat type 2 diabetes mellitus in non-severely obese patients. Obes Surg. 2011;21:1330–1336. doi: 10.1007/s11695-011-0463-5. [DOI] [PubMed] [Google Scholar]
- 42.Kwon Y, Abdemur A, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. The foregut theory as a possible mechanism of action for the remission of type 2 diabetes in low body mass index patients undergoing subtotal gastrectomy for gastric cancer. Surg Obes Relat Dis. 2014;10:235–242. doi: 10.1016/j.soard.2013.09.013. [DOI] [PubMed] [Google Scholar]