Abstract
Background
Danshen is a common traditional Chinese medicine used to treat neoplastic and chronic inflammatory diseases in China. However, the effects of Danshen on human oral cancer cells remain relatively unknown. This study investigated the antiproliferative effects of a Danshen extract on human oral cancer SAS, SCC25, OEC-M1, and KB drug-resistant cell lines and elucidated the possible underlying mechanism.
Methods
We investigated the anticancer potential of the Danshen extract in human oral cancer cell lines and an in vivo oral cancer xenograft mouse model. The expression of apoptosis-related molecules was evaluated through Western blotting, and the concentration of in vivo apoptotic markers was measured using immunohistochemical staining. The antitumor effects of 5-fluorouracil and the Danshen extract were compared.
Results
Cell proliferation assays revealed that the Danshen extract strongly inhibited oral cancer cell proliferation. Cell morphology studies revealed that the Danshen extract inhibited the growth of SAS, SCC25, and OEC-M1 cells by inducing apoptosis. The Flow cytometric analysis indicated that the Danshen extract induced cell cycle G0/G1 arrest. Immunoblotting analysis for the expression of active caspase-3 and X-linked inhibitor of apoptosis protein indicated that Danshen extract-induced apoptosis in human oral cancer SAS cells was mediated through the caspase pathway. Moreover, the Danshen extract significantly inhibited growth in the SAS xenograft mouse model. Furthermore, the Danshen extract circumvented drug resistance in KB drug-resistant oral cancer cells.
Conclusion
The study results suggest that the Danshen extract could be a potential anticancer agent in oral cancer treatment.
Keywords: Danshen, Oral cancer, Drug resistance, Apoptosis
Background
Oral cancer is one of the most common cancers and the leading cause of cancer deaths worldwide [1]. Recent understanding of oral cancer has led to the development of biological therapies using drugs such as 5-fluorouracil (5-FU) and cisplatin [2]. These drugs have shown remarkable activity against difficult-to-treat oral cancer in early clinical trials; however, prolonged drug exposure may result in the development of de novo drug resistance and unexpected side effects, such as allergic reactions, breathing difficulties, swelling, nausea, fever or chills, and dizziness or weakness [3–5]. Therefore, the identification and validation of novel targeted therapies is urgently required to overcome drug resistance and improve patient outcomes.
Danshen (Salvia miltiorrhiza) is a widely used traditional Chinese medicine, which was first described in the Chinese pharmacology book, Shen Nong’s Canon on Materia Medica [6]. Danshen attenuates inflammatory reactions in cardiovascular, hepatic, and tumoral diseases without appreciable adverse effects [6]. Various Danshen extracts contain diterpene quinone and phenolic acid derivatives including tanshinone, cryptotanshinone, isocryptotanshinone, miltirone, tanshinol, salviol, and salvianolic acid B [7–9]. Because of their growth-inhibiting effects on cancer cells [7], Danshen extracts may be suitable as major drug candidates or additional chemotherapeutic agents in oral cancer treatment. In this study, we observed that a Danshen extract (crude) can inhibit human oral cancer SAS, SCC25, OEC-M1, and KB drug-resistant cell lines. It possibly exerts anticancer effects by blocking cell cycle entry into the G1 phase in oral cancer cells.
Methods
Reagents
In this study, 5-FU was purchased from Sigma-Aldrich (F6627); its purity was ≥99%, as determined by high-performance liquid chromatography. The 5-FU was dissolved in saline as a 1.5 mg/mL stock and used as the positive control in an animal model.
Preparation and treatment of the Danshen extract
Danshen (S. miltiorrhiza) was obtained from Dr. Wen-Liang Chang of the National Defense Medical Center in Taipei, Taiwan [10, 11]. Danshen roots were obtained from Chien Yuan Herbal Medicinal Co., Taipei, Taiwan, and identified to be Salviae miltiorrhizae Radix. The pulverized roots (4.5 kg) were extracted with 95% ethanol (15 L) exhaustively for five times. The extract was concentrated by evaporation under reduced pressure. The dried extracts were dissolved in dimethylsulfoxide to prepare a 20 mg/mL stock solution and stored at 4 °C. The Danshen extract was diluted with culture media to achieve the indicated final concentration in each experiment.
Cell culture
The human oral squamous cell carcinoma (OSCC) cell line SAS (JCRB0260) was purchased from the Japanese Collection of Research Bioresources Cell Bank. SCC25 (CRL-1628) was obtained from the American Type Culture Collection (ATCC). OEC-M1 cell line was derived from oral cavity epidermal carcinoma [12], which is a generous gift from Prof. Jenn-Han Chen (National Defense Medical Center, Taiwan). KB drug-resistant cancer cells were purchased from the ATCC (CCL-17; Rockville, MA, USA). KB-7D cells were generated through etoposide (VP-16)-driven selection, which demonstrated topoisomerase II downregulation and multidrug resistance-associated protein overexpression. KB-tax cells were generated through taxol-driven selection. These drug-resistant cancer cells were kindly provided by Dr. Jang-Yang Chang (Cancer Research Division of National Health Research Institutes, Taiwan) [13]. Human oral cancer SAS, SCC25, and OEC-M1 cells were cultured in Roswell Park Memorial Institute 1640 medium. The culture medium was supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 2 mmol/L L-glutamine. The cells were grown at 37 °C in a humidified 5% CO2 incubator.
Cytotoxicity assay
The cells (10,000 cells/well) were cultured in a 24-well plate and then exposed to various concentrations of the Danshen extract for 24 h. The methylene blue dye assay was performed to evaluate the effects of melatonin on cell growth, as described previously [10]. The half-maximal inhibitory concentration (IC50) value resulting from 50% inhibition of cell growth was calculated graphically for comparison with cell growth in controls.
Cell cycle analysis
The cells were harvested with 0.25% trypsin and washed once with phosphate-buffered saline (PBS). After centrifugation, the cells were fixed in 100% ice-cold methanol overnight at −20 °C; next, they were incubated in propidium iodide (50 μg/mL) and RNase (1 mg/mL) for 30 min. Apoptotic cells were identified using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA), and the data were analyzed using CellQuest software. All experiments were performed in triplicate.
Western blot analysis for caspase activity
The cells were lysed directly in an radioimmunoprecipitation assay buffer containing 50 mM Tris (pH 7.8), 0.15 M NaCl, 5 mM ethylenediaminetetraacetic acid, 0.5% Triton X-100, 0.5% NP-40, 0.1% sodium deoxycholate, a protease inhibitor mixture (Calbiochem, Billerica, MA, USA), and a phosphatase inhibitor mixture (Calbiochem, USA). The relative protein concentration in supernatants was determined using a bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL, USA). For immunoblotting, in each lane of 10% sodium dodecyl sulfate–polyacrylamide electrophoresis gel, 30 μg protein from cell lysates was loaded, separated, and transferred onto polyvinyldifluoride membranes (GE Healthcare, UK). These membranes were subsequently probed using specific antibodies against caspase-3 (Cell Signaling, #9662), X-linked inhibitor of apoptosis protein (XIAP; Cell Signaling, #2045), and glyceraldehyde-3-phosphate dehydrogenase (Epitomics, #2251-1).
OSCC animal models
To investigate the effects of the Danshen extract on OSCC in vivo, we used oral cancer SAS xenograft animal models. All experiments were approved by the Institutional Animal Use Committee (IACUC) of the National Defense Medical Center, Taiwan (IACUC 16-022). Eight-week-old nonobese diabetic/severe combined immunodeficiency (NOD/SCID) (NOD.CB17 Prkdc scid/J, National Laboratory Animal Center, Taiwan) mice were maintained in microisolators under specific pathogen-free conditions. These NOD/SCID mice were fed with sterile food and chlorinated sterile water. A total of 12 mice were divided into 3 groups: Danshen extract (10 mg/kg body weight [BW]/d/intraperitoneally [i.p.])-treated; positive control (5-FU, 10 mg/kg BW/d/i.p.); and vehicle control (PBS). Each group of mice was subcutaneously injected with 2 × 106 human oral cancer SAS cells. Drugs were first administered to each group of mice on day 3 prior to tumor palpation, and the treatment was continued until day 32. The size of the transplanted tumors was measured every 3 days using gauged calipers, and the tumor volume was calculated using the following formula: V = 1/2 × (length × width2). At the end of the treatment, the mice were sacrificed, and the tumors were removed, weighed, and photographed.
Haemotoxylin and eosin and immunohistochemical staining
Mice were sacrificed with CO2 inhalation and fixed by perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer. Serial 5-μm histologic sections were deparaffinized in xylene and rehydrated. After blocking of endogenous peroxidase by incubation with 3% hydrogen peroxide, the slides were incubated with anticaspase-3 overnight at 4 °C. The expression levels of targeted proteins were examined by using the antimouse and rabbit peroxidase complex, and peroxidase activity was evaluated using 3-amino-9-ethyl-carbazole. The slides were counterstained with hematoxylin (Sigma) and mounted with mounting solution. Scores were obtained by multiplying the immunohistochemical (IHC) intensity with the percentage of cancer cells stained.
Statistical analysis
The experiments were performed in triplicate. The data for cell proliferation and viability assays are expressed as the mean ± standard deviation. Standard deviations for all measured biological parameters are displayed in the appropriate figures. The Student t test was performed to determine the significance of the differences between the control and treated groups for all experimental test conditions. P < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Results
The growth inhibitory effects of a Danshen extract on human oral cancer SAS, SCC25, and OEC-M1 cells were first demonstrated by conducting microscopic studies. The results showed that the Danshen extract effectively inhibited the growth of both tongue cancer cells (SAS and SCC25) and gingival cancer cells (OEC-M1) (Fig. 1). The antiproliferative effects of the Danshen extract on human oral cancer cells were further confirmed by proliferation assays (Fig. 2). The methylene blue proliferation assay revealed that Danshen treatment (5–10 μg/mL) could result in an approximately 50% reduction in oral cancer cell proliferation (Fig. 2).
Fig. 1.
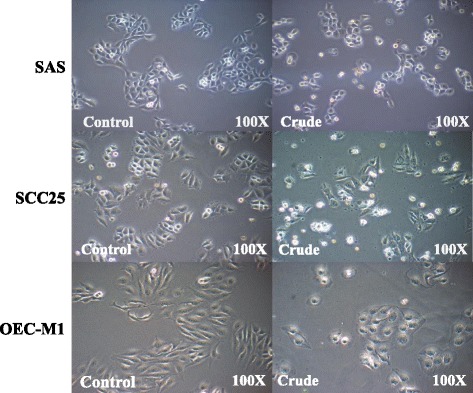
Danshen crude induced morphological change in oral cancer cells. Effect of the Danshen crude on morphological changes in SAS, SCC25, and OEC-M1 oral cancer cell lines. Original magnification, 100×
Fig. 2.
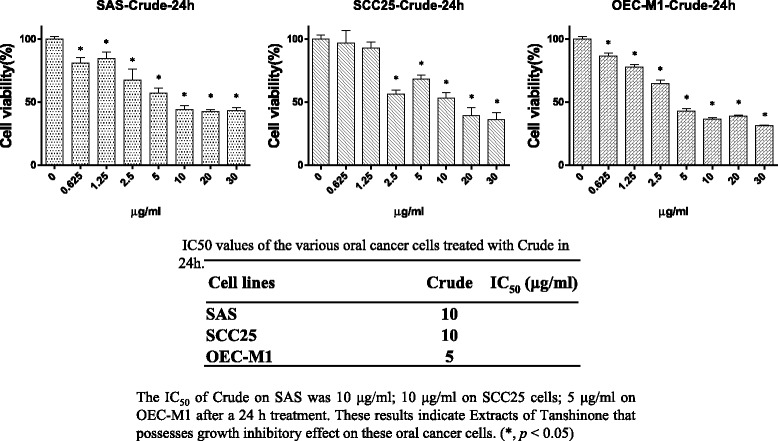
Inhibition of oral cancer cell (SAS, SCC25, and OEC-M1) growth in vitro via the Danshen crude. The antioral cancer efficacy of the Danshen crude on three oral cancer cell lines (SAS, SCC25, and OEC-M1) treated for 24 h. The extract significantly reduced the cell viability of all three OSCC lines in a dose-dependent manner
To examine whether the Danshen extract inhibited cell proliferation by cell cycle arrest, cell cycle profiling of the SAS cells was performed. The cells were treated with the Danshen extract (20 μg/mL) for 24 h and their cell cycle profiles were analyzed using flow cytometry. The analysis results revealed that the Danshen extract engendered an increase in the G0/G1 phase, with a concomitant reduction in the S phase, in the SAS cells (Fig. 3). In addition, the sub-G1 apoptotic dead cell population increased after Danshen treatment (Fig. 3). These results suggest that the Danshen extract induced oral cancer cell apoptosis and cell cycle G0/G1 arrest.
Fig. 3.
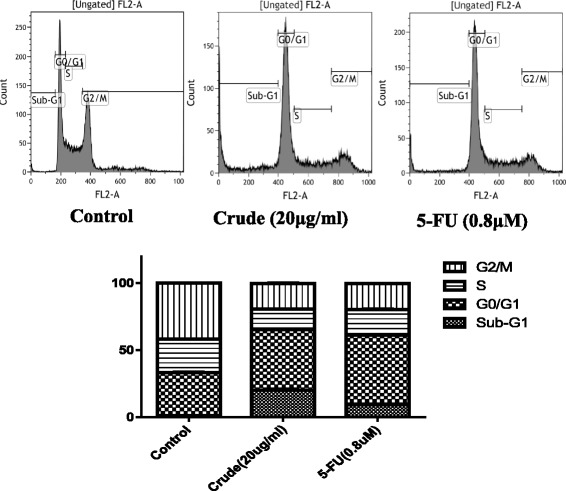
Oral cancer G0/G1 arrest induced by the Danshen crude. Flow cytometry analysis results of crude- and 5-FU-treated SAS cells. The cells were treated with extract (20 μg/mL) and 5-FU (0.8 μM) for 24 h. Harvested cells were stained with propidium iodide
To gain a clearer understanding of the antioral cancer mechanism in Danshen-extract-treated cancer cells, we investigated the effects of the Danshen extract on the expression levels and activities of intracellular signaling molecules (Fig. 4). Specifically, the expression levels of the apoptotic protein caspase-3 and antiapoptotic protein XIAP were upregulated and downregulated, respectively, in Danshen-extract-treated cancer cells (Fig. 4). To delineate the antiproliferative effects of the Danshen extract, the SAS cells were treated with various concentrations of the extract for different treatment periods. In addition, Western blotting was performed to examine the effects of the Danshen extract on intracellular signaling molecules. The results (Fig. 4) demonstrated that the Danshen extract effectively suppressed XIAP expression and upregulated caspase-3 expression.
Fig. 4.
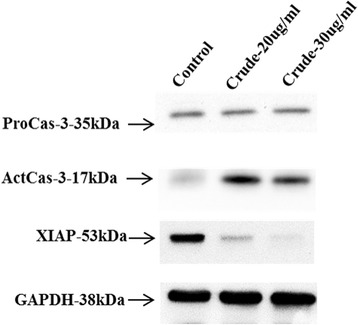
Apoptosis in oral cancer cells induced by the Danshen crude. Lysates of the SAS cells treated with extract (20 and 30 μg/mL) for 24 and 48 h were subjected to an immunoblot analysis with antibodies against caspase-3 and XIAP. GAPDH served as the loading control
The antioral cancer effects of the Danshen extract were further investigated in the SAS solid tumor xenografts of immunodeficient mice. The daily treatments were initiated a day after the SAS cells were transplanted into the NOD/SCID mice. The doses for individual treatments (for 32 d) are outlined as follows: Danshen extract, 10 mg/kg/d/i.p.; 5-FU, 10 mg/kg/d/i.p. During this period, each mouse was manually examined for tumor volume at least four times. The Danshen extract and 5-FU (i.e., the first-line drug for oral cancer treatment) significantly inhibited tumor growth in the NOD/SCID mice, and the bodies weight of the mice were not changed (Fig. 5).
Fig. 5.
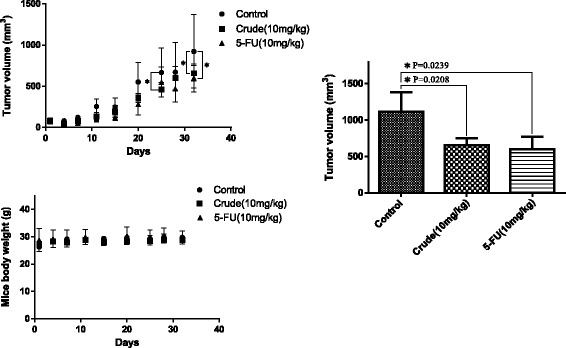
Repression of tumor cell growth in SAS xenograft mice via the Danshen crude. The Danshen extract inhibited oral cancer cell growth in vivo. The SAS xenograft was exposed to either saline (control) or the Danshen extract (crude, 10 mg/kg), and 5-FU (10 mg/kg) served as the positive control
Histologic sections obtained from the SAS xenografts were used to evaluate the antiproliferative effects of the Danshen extract in SAS cells. The expression levels of caspase-3 (apoptotic biomarker) were then analyzed through IHC staining (Fig. 6), which our statistical analysis showed were significantly higher in the Danshen-extract-treated group than in the untreated control groups.
Fig. 6.
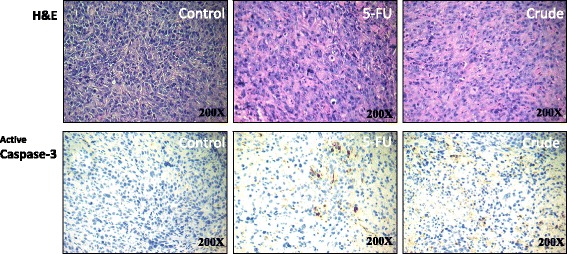
Active caspase-3 expression induced in oral cancer cells in the SAS xenografts model using the Danshen crude. H&E staining and IHC were performed after administration of the Danshen crude, 5-FU, or PBS (a vehicle control). The SAS xenografts model stained active caspase-3. Immunodetectable proteins are stained brown and the nuclei are counterstained blue. Original magnification, 200×
The Danshen extract also significantly inhibited the proliferation of KB drug-resistant cells, indicating that the treatment had circumvented drug resistance in these cells (Fig. 7).
Fig. 7.
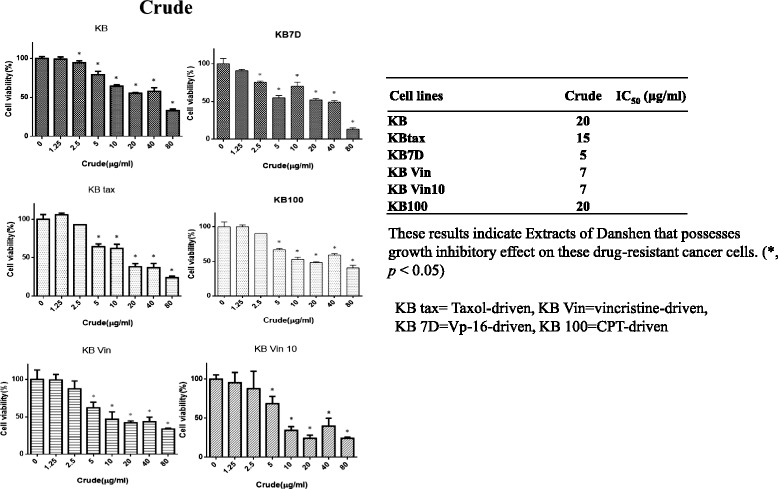
Inhibition of drug-resistant KB cell growth in vitro via the Danshen crude. An assessment of cell proliferation and viability by using the methylene blue assay in six drug-resistant oral cancer cells treated with varying concentrations of the Danshen crude (0–80 μg/mL) or dimethyl sulfoxide (1 μL/mL) for 24 h
Discussion
The prevalence of oral cancer has increased globally in recent years [14], and 5-FU-based chemotherapy has been widely used to reduce the risk of relapse after surgery. The 5-FU plus docetaxel treatment, with the addition of oxaliplatin chemotherapy (which improves survival significantly compared with 5-FU alone [15]), has been widely accepted as the standard adjuvant chemotherapy for OSCC. However, inflammation, neutropenia and lymphopenia, which are common chemotherapy-induced toxicities, may influence the prognosis of adjuvant chemotherapy in cancer treatment [16, 17]. By contrast, Danshen is a natural compound that has anti-inflammation and anticancer effects, which can be effective for the supportive care of cancer patients [18]. Some clinical studies have indicated that bioactive natural compounds play a key role in the treatment of many cancers [6, 19]. In the present study, a Danshen extract was observed to inhibit oral cancer cell proliferation (Figs. 1 and 2) through cell cycle G0/G1 arrest (Fig. 3).
Apoptosis is a well-defined self-suicidal process to inhibit tumor growth. Many studies have reported that chemotherapeutic drugs exert antitumor effects by triggering apoptosis through various molecular mechanisms [20]. One previous study revealed that tanshinone IIA induces apoptosis in human oral cancer KB cells through a mitochondria-dependent pathway [8]. Our results demonstrate that the Danshen extract upregulated caspase-3 expression and repressed XIAP expression in SAS cells. In addition, our results confirm the involvement of apoptosis in Danshen-induced in vitro and in vivo growth inhibition in human oral cancer cells (Figs. 4, 5 and 6).
Cell cycle dysregulation results in uncontrolled cell growth, which can lead to cancer development [21]. Therefore, the targeted detection of cell-cycle-related errors in cancer cells is considered a potential strategy for tumor growth control [22]. Research has revealed that the leading bioactive components (salvianolic acid B [23] and tanshinone IIA [8]) of Danshen result in increased G0/G1 and G2/M phases in oral cancer cells, and in head and neck cancer cells, respectively. The present study revealed cell cycle G0/G1 arrest in Danshen-extract-treated SAS cells and an increased sub-G1 apoptotic dead cell population (Fig. 3). These findings suggest that the Danshen extract induces cell cycle G0/G1 arrest and apoptosis.
Resistance to chemotherapy and molecular-targeted therapies is a major concern in current cancer research. Our results reveal that the Danshen extract suppressed growth in drug-resistant oral cancer cells (including taxol- [24], vincristine- [25], Vp-16- [26], and camptothecin- [27] resistant cells; Fig. 7). These results suggest that the Danshen extract may be a potential novel chemotherapeutic agent in oral cancer treatment.
Conclusions
In conclusion, this in vitro and in vivo study revealed that a Danshen extract exerts antiproliferative effects in human oral cancer cells and KB drug-resistant cells through multiple pathways, such as the induction of apoptosis. Therefore, Danshen treatment should be considered a novel approach for drug-resistant oral cancer prevention and treatment.
Acknowledgements
The authors acknowledge the technical services provided by Instrument Center of National Defense Medical Center and the laboratory animal center of National Defense Medical Center.
Funding
Tri-Service General Hospital, Taiwan, Republic of China (grants No. TSGH-C105-006-008-S05, TSGH-C106-004-006-008-S05, TSGH-C105-190, TSGH-C106-121, Ministry of National Defense, Republic of China (grants No. MAB-106-090) and National Science Council, Taiwan, Republic of China (grants No. MOST 105-2314-B-016-021-MY3) have jointly funded this work.
Availability of data and materials
All data and materials are contained and described within the manuscript.
Abbreviations
- NOD/SCID
Nonobese diabetic/severe combined immunodeficiency
- OSCC
Oral squamous cell carcinoma
- XIAP
X-linked inhibitor of apoptosis protein
Authors’ contributions
CYY and CCH contributed equally to this work. CYY and CCH performed the biochemical and animal study, analyzed and interpreted the data, designed the study, and drafted the manuscript. CKL and CSL analyzed the IHC data. BP assisted with the animal study. GJL and HKST designed the study and interpreted the data. WLC provided the Danshen extract and reagents. YWC designed the study, interpreted the data, and critically revised the manuscript. All authors read and approved the final manuscript.
Ethics approval
Animal study: The animal use protocol listed below has been reviewed and approved by the Institutional Animal Use Committee (IACUC), National Defense Medical Center, Taipei, Taiwan (No: IACUC 16-022).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cheng-Yu Yang, Email: hslcuyang@gmail.com.
Cheng-Chih Hsieh, Email: p75phr60@gmail.com.
Chih-Kung Lin, Email: doc31795@yahoo.com.tw.
Chun-Shu Lin, Email: chunshulin@gmail.com.
Bo Peng, Email: t022134@seed.net.tw.
Gu-Jiun Lin, Email: lingujiun@mail.ndmctsgh.edu.tw.
Huey-Kang Sytwu, Email: sytwu@ndmctsgh.edu.tw.
Wen-Liang Chang, Email: wlchang@ndmctsgh.edu.tw.
Yuan-Wu Chen, Phone: +886287923100-18521, Email: h6183@yahoo.com.tw.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Ramadas K, Amarasinghe H, Subramanian S, Johnson N. Oral cancer: prevention, early detection, and treatment. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Cancer: disease control priorities, third edition (Volume 3). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2015 Nov. Chapter 5. [PubMed]
- 3.Cooley ME, Davis L, Abrahm J. Cisplatin: a clinical review. Part II--nursing assessment and management of side effects of cisplatin. Cancer Nurs. 1994;17(4):283–293. doi: 10.1097/00002820-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Xu R, Wang Q. Large-scale automatic extraction of side effects associated with targeted anticancer drugs from full-text oncological articles. J Biomed Inform. 2015;55:64–72. doi: 10.1016/j.jbi.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moschovi M, Critselis E, Cen O, Adamaki M, Lambrou GI, Chrousos GP, Vlahopoulos S. Drugs acting on homeostasis: challenging cancer cell adaptation. Expert Rev Anticancer Ther. 2015;15(12):1405–1417. doi: 10.1586/14737140.2015.1095095. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Guo J, Bao J, Lu J, Wang Y. The anticancer properties of salvia miltiorrhiza Bunge (Danshen): a systematic review. Med Res Rev. 2014;34(4):768–794. doi: 10.1002/med.21304. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Ge PJ, Jiang L, Li FL, Zhu QY. Modulation of growth and angiogenic potential of oral squamous carcinoma cells in vitro using salvianolic acid B. BMC Complement Altern Med. 2011;11:54. doi: 10.1186/1472-6882-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng PY, Lu WC, Hsieh MJ, Chien SY, Chen MK. Tanshinone IIA induces apoptosis in human oral cancer KB cells through a mitochondria-dependent pathway. Biomed Res Int. 2014;2014:540516. doi: 10.1155/2014/540516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Zhao Y, Guo Y, Gu X. Salvianolic acid B, a potential chemopreventive agent, for head and neck squamous cell cancer. J Oncol. 2011;2011:534548. doi: 10.1155/2011/534548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu WL, Chang WL, Chen CF. Cytotoxic activities of tanshinones against human carcinoma cell lines. Am J Chin Med. 1991;19(3–4):207–216. doi: 10.1142/S0192415X91000284. [DOI] [PubMed] [Google Scholar]
- 11.Tsai H-T, Chang W-L, Tu H-P, Fu E, Hsieh Y-D, Chiang C-Y. Effects of salvia miltiorrhiza ethanolic extract on lipopolysaccharide-induced dental alveolar bone resorption in rats. J Dent Sci. 2016;11(1):35–40. doi: 10.1016/j.jds.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CY, Meng CL. Regulation of PG synthase by EGF and PDGF in human oral, breast, stomach, and fibrosarcoma cancer cell lines. J Dent Res. 1994;73(8):1407–1415. doi: 10.1177/00220345940730080301. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CC, Hsieh HP, Pan WY, Chen CP, Liou JP, Lee SJ, Chang YL, Chen LT, Chen CT, Chang JY. BPR0L075, a novel synthetic indole compound with antimitotic activity in human cancer cells, exerts effective antitumoral activity in vivo. Cancer Res. 2004;64(13):4621–4628. doi: 10.1158/0008-5472.CAN-03-3474. [DOI] [PubMed] [Google Scholar]
- 14.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 15.Tamatani T, Ferdous T, Takamaru N, Hara K, Kinouchi M, Kuribayashi N, Ohe G, Uchida D, Nagai H, Fujisawa K, et al. Antitumor efficacy of sequential treatment with docetaxel and 5-fluorouracil against human oral cancer cells. Int J Oncol. 2012;41(3):1148–1156. doi: 10.3892/ijo.2012.1544. [DOI] [PubMed] [Google Scholar]
- 16.El-Sayyad HI, Ismail MF, Shalaby FM, Abou-El-Magd RF, Gaur RL, Fernando A, Raj MH, Ouhtit A. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int J Biol Sci. 2009;5(5):466–473. doi: 10.7150/ijbs.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamano T, Takayasu Y, Nakao N, Kubota A. Evaluation of hepatic toxicity following high-dose 5-FU arterial infusion chemotherapy: analysis of 42 cases of colorectal liver metastases. Nihon Igaku Hoshasen Gakkai Zasshi. 2000;60(3):94–102. [PubMed] [Google Scholar]
- 18.Stumpf C, Fan Q, Hintermann C, Raaz D, Kurfurst I, Losert S, Pflederer W, Achenbach S, Daniel WG, Garlichs CD. Anti-inflammatory effects of danshen on human vascular endothelial cells in culture. Am J Chin Med. 2013;41(5):1065–1077. doi: 10.1142/S0192415X13500729. [DOI] [PubMed] [Google Scholar]
- 19.Choi JG, Eom SM, Kim J, Kim SH, Huh E, Kim H, Lee Y, Lee H, Oh MS. A comprehensive review of recent studies on herb-drug interaction: a focus on Pharmacodynamic interaction. J Altern Complement Med. 2016;22(4):262–279. doi: 10.1089/acm.2015.0235. [DOI] [PubMed] [Google Scholar]
- 20.Hu W, Kavanagh JJ. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003;4(12):721–729. doi: 10.1016/S1470-2045(03)01277-4. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Visconti R, Della Monica R, Grieco D. Cell cycle checkpoint in cancer: a therapeutically targetable double-edged sword. J Exp Clin Cancer Res. 2016;35(1):153. doi: 10.1186/s13046-016-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao Y, Xie T, Korotcov A, Zhou Y, Pang X, Shan L, Ji H, Sridhar R, Wang P, Califano J, et al. Salvianolic acid B inhibits growth of head and neck squamous cell carcinoma in vitro and in vivo via cyclooxygenase-2 and apoptotic pathways. Int J Cancer. 2009;124(9):2200–2209. doi: 10.1002/ijc.24160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22(47):7280–7295. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohno K, Kikuchi J, Sato S, Takano H, Saburi Y, Asoh K, Kuwano M. Vincristine-resistant human cancer KB cell line and increased expression of multidrug-resistance gene. Jpn J Cancer Res. 1988;79(11):1238–1246. doi: 10.1111/j.1349-7006.1988.tb01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long BH, Wang L, Lorico A, Wang RC, Brattain MG, Casazza AM. Mechanisms of resistance to etoposide and teniposide in acquired resistant human colon and lung carcinoma cell lines. Cancer Res. 1991;51(19):5275–5283. [PubMed] [Google Scholar]
- 27.Beretta GL, Gatti L, Perego P, Zaffaroni N. Camptothecin resistance in cancer: insights into the molecular mechanisms of a DNA-damaging drug. Curr Med Chem. 2013;20(12):1541–1565. doi: 10.2174/0929867311320120006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are contained and described within the manuscript.


