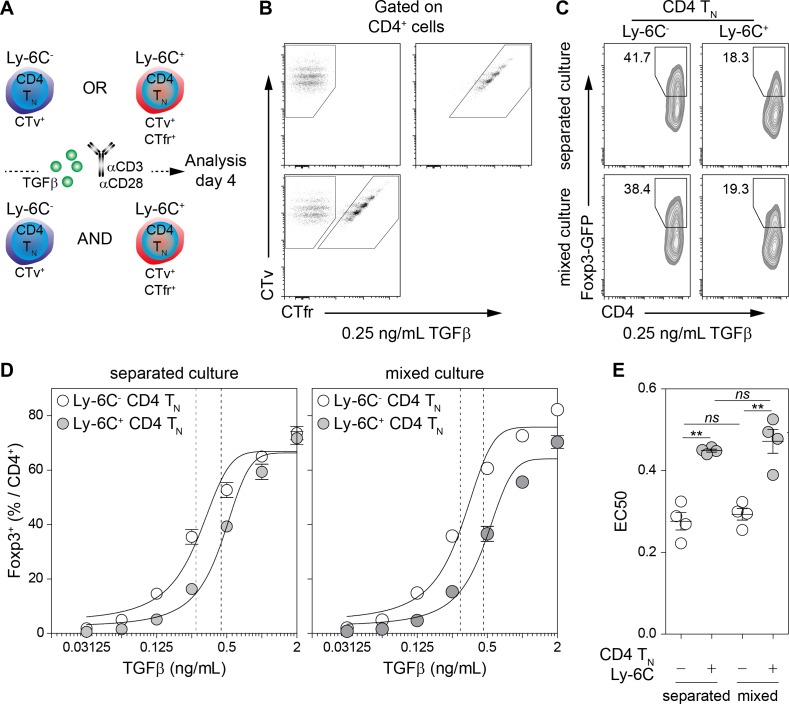
Figure 1. Cell-intrinsic enhanced ability of Ly-6C- CD4 TN cells to commit into iTreg cells.
(A–E) Flow-cytometry sorted Ly-6C- and Ly-6C+ CD4 TN cells from C57BL/6 Foxp3-GFP mice were stained with CTv (Ly-6C-) or CTv and CTfr (Ly-6C+) and stimulated separately or together for 4 days with coated αCD3 and αCD28 Abs (4 µg/mL), in the presence of graded doses of TGFβ1. (A) Diagram illustrating the experimental protocol. (B) Representative CTv/CTfr dot-plots for gated CD4+ cells recovered after 4 days of culture. Ly-6C- and Ly-6C+ CD4 TN cells were either cultured separately (top left and right panels, respectively) or together (bottom panel) (C) Representative Foxp3/CD4 contour-plots and proportions of Foxp3+ cells for gated CD4+ cells are shown at a dose of 0.25 ng/mL TGFβ1. (D) Proportions of Foxp3+ cells among CD4+ cells are shown as a function of TGFβ1 concentration. Mean ± s.e.m of four independent experiments are shown. (E) Concentrations of TGFβ1 needed to obtain 50% of the maximal percentages of iTreg-cell polarization (EC50) were calculated for each CD4 TN cell subset in separated or mixed cultures. Each dot represents an independent experiment. Significance of differences were assessed using a two-tailed paired Student’s t-test. Values of p<0.05 were considered as statistically significant (**p<0.01; ns, not significant).